Probing Photocatalytic Hydrogen Evolution of Cobalt Complexes: Experimental and Theoretical Methods
WU Hai-Su, MIAO Ti-Fang*, SHI Hai-Xia, XU Yun*, FU Xian-Liang and QIAN Li
Chin. J. Struct. Chem. 2021, 40, 1696-1709 DOI: 10.14102/j.cnki.0254-5861.2011-3239
December 15, 2021
hydrogen production, cobalt complex, photocatalysis, theoretical calculation
ABSTRACT
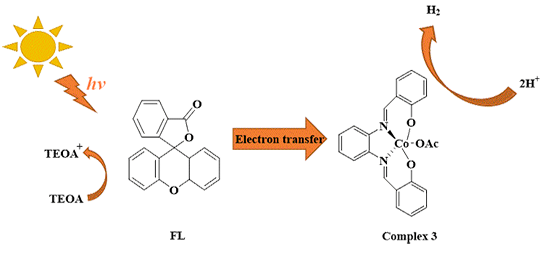
This work reports on the synthesis and characterization of a series of Schiff-base cobalt(Ⅲ) complexes 1~4 that exhibit an obvious catalytic activity for hydrogen production in aqueous solution using
fluorescein (FL) and triethanolamine (TEOA) as photosensitizer and electron donor,
respectively. The complexes display the capability of splitting of water for H2 evolution. Under optimized conditions,
complex 3 shows better properties for
photocatalysis, 25 mg of which can release 152.3 μmol of H2 after irradiation for 3 h. The mechanism for light-driven H2 production
was explored by experiments and density
functional theory (DFT). Meanwhile, the reason of releasing hydrogen was explained theoretically
in detail. The research results will
help to understand the interaction of cobalt
complexes with the photosensitizer and design new photocatalysis
for the future.