Syntheses and Crystal Studies of Novel C-3΄-N-sulfonyl and 7-O-acyl Modified Paclitaxel Analogues
XIAO Shang-Qing, CUI Yong-Mei, QIU Wei-Qing, CUI Ye-Sha and LIN Hai-Xia*
Chin. J. Struct. Chem. 2021, 40, 1088-1097 DOI: 10.14102/j.cnki.0254-5861.2011-3116
August 15, 2021
paclitaxel, 3΄-N-sulfonyl, crystal structure, bioactivity, conformation
ABSTRACT
Three C-3΄-N-sulfonyl and C-7-O-acyl paclitaxel analogues were synthesized from
10-deacetyl baccatin III
(10-DAB) and their structures
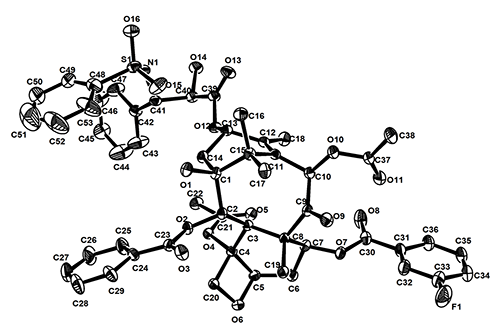
were confirmed by 1H NMR, 13C NMR and HR-MS. Among them, the crystal structure
of compound 9b was determined by single-crystal X-ray diffraction. Compound 9b crystallizes in monoclinic system, space group P21 with a = 12.395(4), b = 15.215(5), c = 14.905(5) Å, β =
105.559(4)° and Z = 2. In the
structure, the introduction of the hydrophobic 3-fluorobenzoyl group
at C(7) has little effect on the conformation of tetracyclic system. However, the conformation of the side-chain at C(13) in 9b is quite different from that
of paclitaxel and docetaxel.