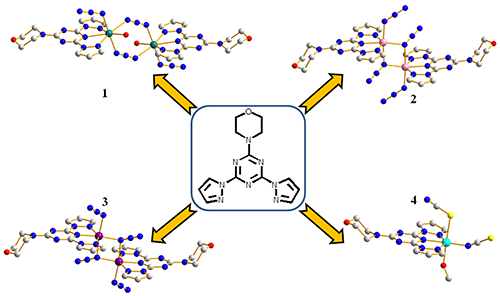
Four
Complexes of Mn(II), Co(II), Ni(II) and Cu(II) Based on 2-Morpholine-4-yl-4,6-di-pyrazol-1-yl-1,3,5-triazine
TIAN Wen-Yu and CHU Jin-Feng*
Chin. J. Struct. Chem. 2021, 40, 729-738 DOI: 10.14102/j.cnki.0254-5861.2011-3033
June 15, 2021
azide bridged complexes, supramolecular, crystal structure, thiocyanate
ABSTRACT
Three azide bridged complexes, namely, [Mn2L2(N3)4(H2O)2]
(1), [Co2L2(N3)4]·(H2O)3 (2) and [Ni2L2(N3)3(H2O)]N3·(H2O)4 (3) (L = 2-morpholine-4-yl-4,6-di-pyrazol-1-yl-1,3,5-triazine),
were synthesized by the reaction of L ligand, sodium azide with Mn(II), Co(II)
and Ni(II) chlorides. The copper(II) chloride combined with thiocyanate and L
ligand to form a mononuclear complex [CuL(CH3OH)(SCN)(NCS)] (4). Complexes 1~4 were characterized by IR,
elemental analysis and X-ray crystallographic analysis. It was worth noting
that two Mn(II) atoms were connected by the end-to-end mode in 1, while Co(II) and Ni(II) atoms were
connected by the end-on mode in 2 and 3. In complex 4, the central copper atom was
coordinated with a sulfur atom and a nitrogen atom of the two thiocyanate
ligands, respectively. Hydrogen bonds, π-π stacking interactions,
thermogravimetric analysis and fluorescence properties of 1~4 were studied.