Syntheses, Structures and Anticancer Activities of
Two Tri(o-halobenzyl)tin Substituted
Benzoates
TAN Xu-Liang, ZHANG Fu-Xing*, HE Li-Fang, GUI Shi-Yin, ZHANG Yi-Ling, ZHU Xiao-Ming, SHENG Liang-Bing, FENG Yong-Lan, YU Jiang-Xi and JIANG Wu-Jiu
Chin. J. Struct. Chem. 2021, 40, 675-681 DOI: 10.14102/j.cnki.0254-5861.2011-3080
May 15, 2021
tri(o-chlorobenzyl)tin 2,4,6-trimethylbenzoate, tri(o-bromobenzyl)tin salicylate, crystal structure, quantum chemistry, in vitro antitumor activity
ABSTRACT
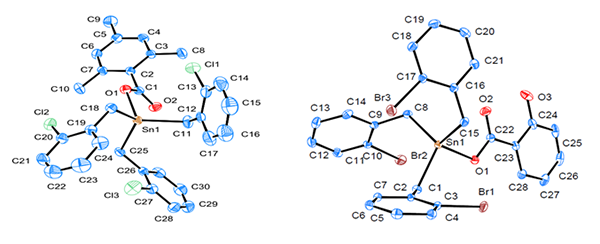
Tri(o-chlorobenzyl)tin
2,4,6-trimethylbenzoate (1) and tri(o-bromobenzyl)tin salicylate (2) were synthesized and characterized
by elemental analysis, IR spectroscopy, NMR (1H, 13C and 119Sn),
thermogravimetric analysis, and single-crystal X-ray diffraction. The initio calculation and in vitro anticancer activity test were
performed for compounds 1 and 2. They are both single tin nucleus
structures and the tin atoms were tetracoordinated in a distorted tetrahedral
configuration; Compounds 1 and 2 showed stronger anticancer activity
than cisplatin in human cervical cancer cells (Hela), liver cancer cells (HuH-7),
non-small cell lung cancer cells (A549), lung adenocarcinoma cells (H1975) and
breast cancer (MCF-7).