Two New Copper Complexes by H4AQTC (Anthraquinone-1,4,5,8-tetracarboxylic Acid): Syntheses, Structures and Properties
YAN Wei-Hong* and ZENG Xian-Cai
Chin. J. Struct. Chem. 2021, 40, 349-356 DOI: 10.14102/j.cnki.0254-5861.2011-2923
March 15, 2021
anthraquinone-1,4,5,8-tetracarboxylic acid, di-2-pyridyl ketone, syntheses, crystal structure
ABSTRACT
Two
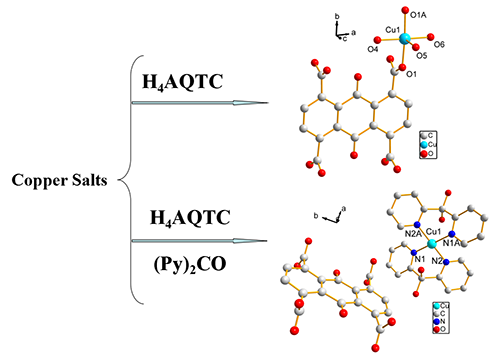
new complexes [Cu(AQTC)0.5(H2O)3]·3H2O}n (1, H4AQTC =
anthraquinone-1,4,5,8-tetracar- boxylic acid) and Cu[(Py)2C(OH)2]2(H2AQTC)·2H2O (2, (Py)2CO = di-2-pyridyl ketone)
have been prepared and characterized by elemental analyses and IR spectroscopy.
X-ray crystallographic studies show that complex 1 crystallizes in monoclinic space group C2/m and complex 2 in
monoclinic space group P21/c. Complex 1 features a
1D chain structure by carboxyl oxygen atoms. Complex 2 displays a mononuclear structure and anions and cations are separated. What's interesting is that the
ligand of H4AQTC with eight carboxyl oxygen atoms and two quinone
oxygen atoms does not directly coordinate with metals, and only exist as a
counter-anion in complex 2. Three-dimensional structures of two
complexes are formed by intermolecular interactions. The thermogravimetric
analyses of two complexes are investigated. The luminescent properties of complex 1 are investigated as well.