Porous
Iron- and Cobalt-based Single Crystals with Enhanced Electrocatalysis
Performance
ZHANG Fei-Yan and XIE Kui*
Chin. J. Struct. Chem. 2021, 40, 61-69 DOI: 10.14102/j.cnki.0254-5861.2011-2745
January 15, 2021
porous single crystal, crystal growth, electrocatalysis
ABSTRACT
Porous
single crystals have the characteristics of long-range ordering structure and
large specific surface area, which will significantly enhance their
electrochemical performance. Here, we report a method different from the
conventional porous single crystal growth method. This method is to directly
convert single crystal precursors Co3O4 and Fe3O4 into Co2N and Fe2N, and then further reduces them to
porous single crystals Co and Fe particles under H2/Ar atmosphere.
The removal of O2– in the lattice channel at the pressure of 25~300 torr and the
temperature of 300~600 °C will promote nitridation
of the single-crystalline Co–O and Fe–O frames, and further remove N3– in H2/Ar atmosphere and recrystallize as Co and Fe. These porous
single crystals exhibit enhanced electrochemical properties due to their
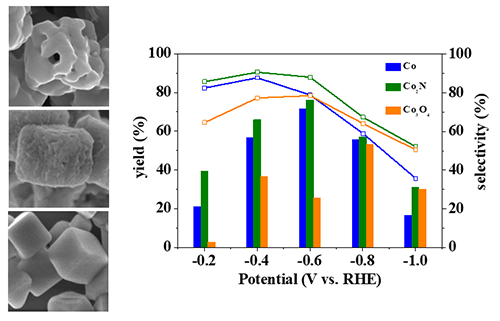
structural coherence and highly active surface. We demonstrated
that the aminobenzene yield was up to 91% and the selectivity reached 92% in
the electrochemical reduction of nitrobenzene.