Cover Picture
Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries
Renshu Huang, Jinli Chen, Xingfa Chen, Tianqi Yu, Huyi Yu, Kaien Li, Bin Li*, Shibin Yin* Submit a Manuscript
Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries
Renshu Huang, Jinli Chen, Xingfa Chen, Tianqi Yu, Huyi Yu, Kaien Li, Bin Li*, Shibin Yin* Submit a Manuscript
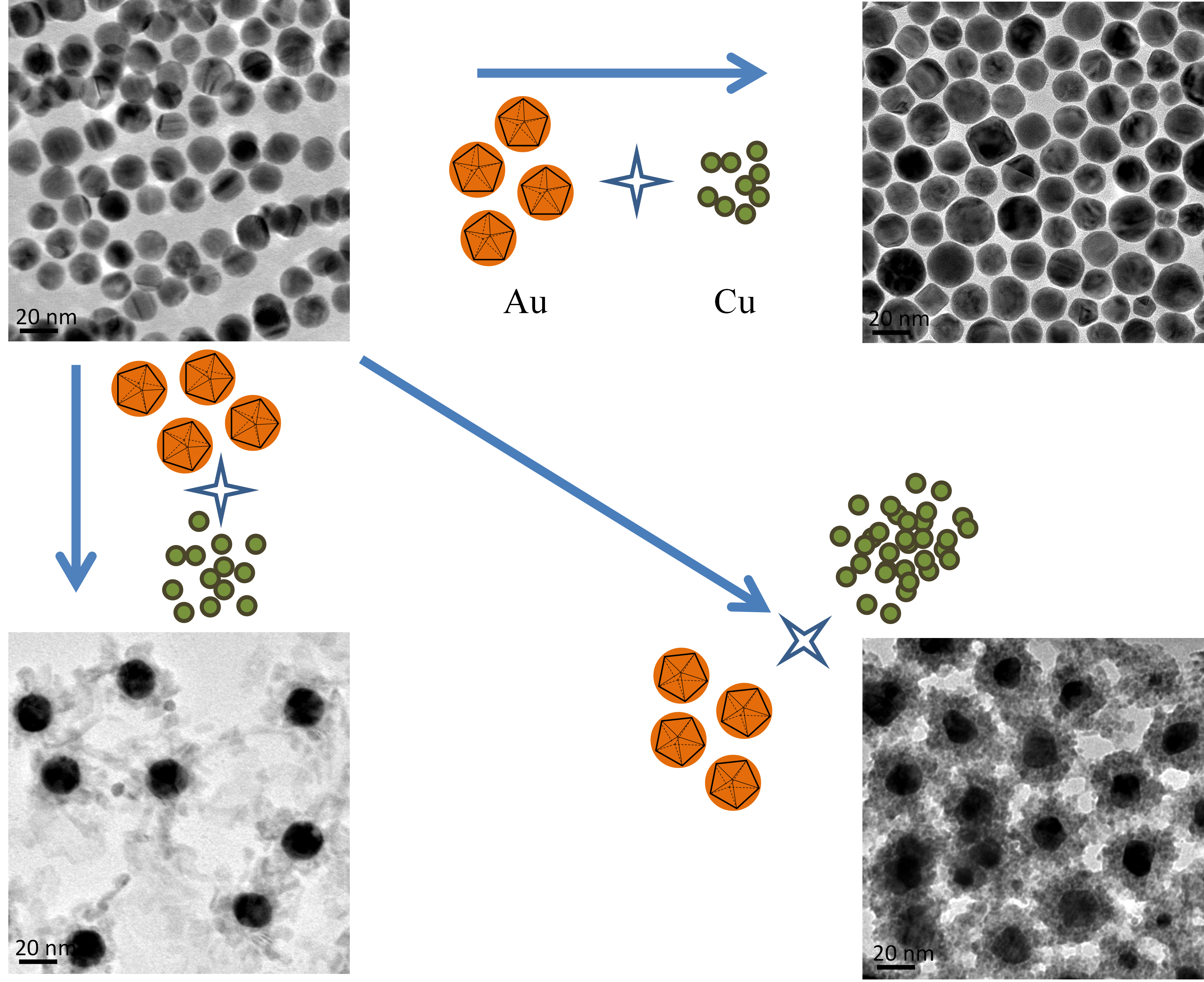
Core-shell gold-copper nanoparticles: Evolution of copper shells on gold cores at different gold/copper precursor ratios
Shaonan Tian, Yu Zhang, Qing Zeng, Junyu Zhong, Hui Liu*, Lin Xu*, Jun Yang*
Chin. J. Struct. Chem., 2023, 42: 100160. DOI: 10.1016/j.cjsc.2023.100160
November 15, 2023
Core-shell nanostructure; Seed-mediated growth; Electronic Interaction; Lattice strain; Methanol oxidation reaction
ABSTRACT
Core-shell nanostructures usually exhibit tunable catalytic properties in comparison with their single core or shell counterpart due to electronic interaction and lattice strain between the core and shell regions. Herein, we report the intriguing evolution of copper (Cu) shells on the gold (Au) cores at different Au/Cu precursor ratios during the synthesis of core-shell Au–Cu nanoparticles at an organic medium via seed-mediated growth method. In brief, at relatively low Cu ratios, quasi-spherical Au–Cu nanoparticles with conventional core-shell structures are the majority products, in which the Cu shell thickness increases with the increase of Cu precursor ratios. The difference is that at high Cu ratios, the Cu shells no longer increase their thickness, but evolve into a dendritic structure. Interestingly, the core-shell Au–Cu nanoparticles with dendritic Cu shells could be transformed into interesting Au–Cu cage-bell structures after a ripening process at elevated temperature. Further, through galvanic replacement reaction with Pt precursors, the thin Cu shells could be converted into CuPt alloy shells on the Au cores, which exhibit enhanced activity towards methanol oxidation reaction with satisfactory durability, in comparison with that of commercial Pt/C catalysts.