Cover Picture
Submit a Manuscript
Magnetic
field-assisted microbial corrosion construction ironsulfides incorporated
nickel-iron hydroxide towards efficientoxygen evolution
Xianzheng Zhang, Yana Chen, Zhiyong Ye, Huilin Hu, Ling Lei, Feng You, Junlong Yao, Huan Yang*, Xueliang Jiang*
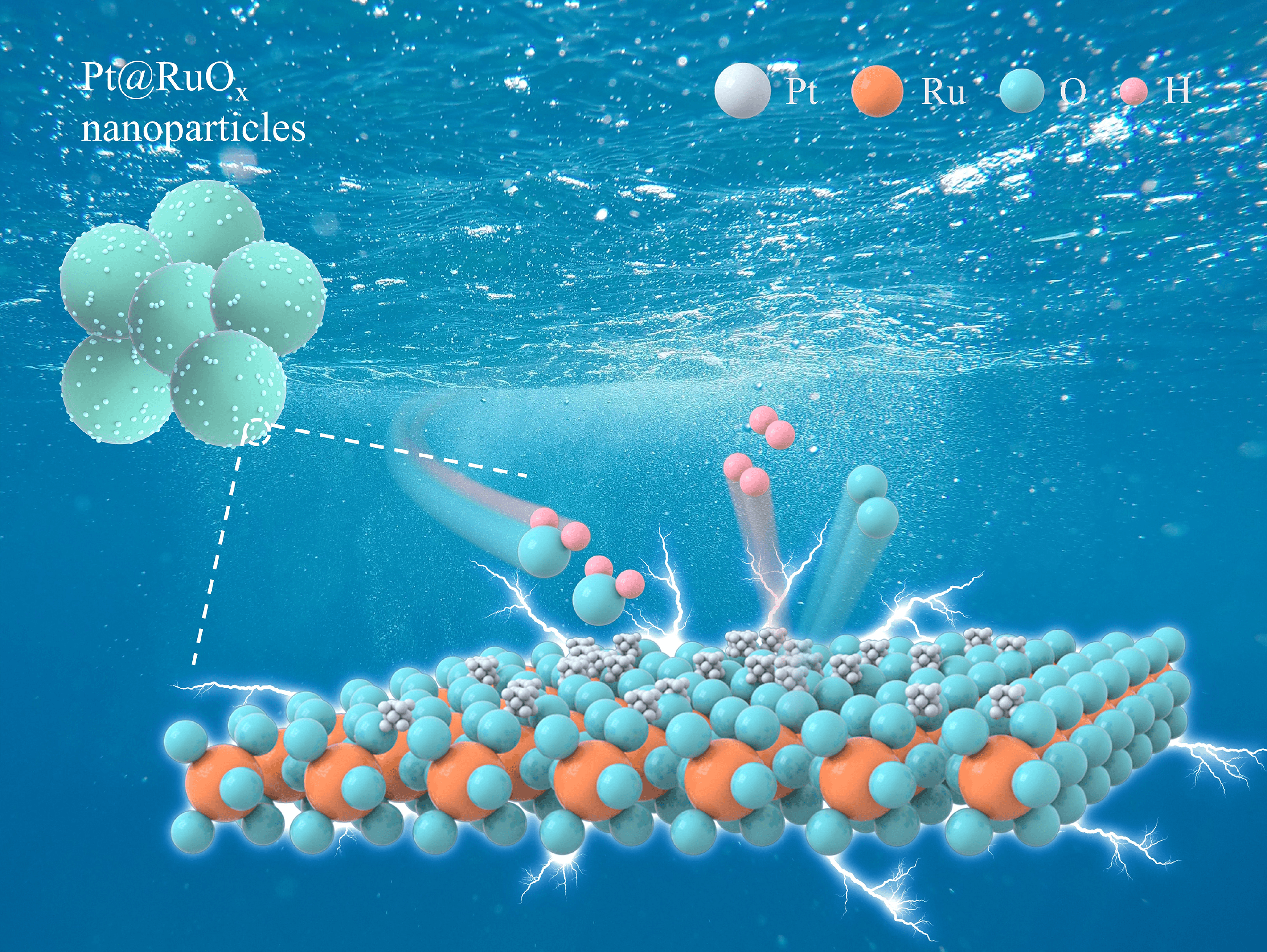
Nanostructured Pt@RuOx catalyst for boosting overall acidic seawater splitting
Zimo Peng, Quan Zhang, Gaocan Qi*, Hao Zhang*, Qian Liu, Guangzhi Hu, Jun Luo, Xijun Liu*
Chin. J. Struct. Chem., 2024, 43: 100191. DOI: 10.1016/j.cjsc.2023.100191
January 15, 2024
Acidic water splitting; Bifunctional catalysts; Pt@RuOx nanoparticles; Synergistic effect
ABSTRACT
In the domain of acidic water splitting, designing bifunctional catalysts that marry high activity with enduring stability is a formidable challenge. Herein, we have constructed platinum-containing ruthenium oxide nanoparticles (Pt@RuOx NPs) to achieve excellent overall water splitting performance in acidic electrolytes. Pt@RuOx NPs demonstrate exceptional catalytic activity for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in acidic seawater, requiring overpotentials of only 20 and 157 mV, respectively to achieve a current density of 10 mA cm−2. Furthermore, in 0.5 M H2SO4, this catalyst exhibits high HER (19 mV) and OER (236 mV) catalytic activities. The two-electrode water splitting system composed of bifunctional Pt@RuOx NPs requires cell voltages of only 1.442 and 1.465 V to deliver a current density of 10 mA cm−2 in acidic seawater and 0.5 M H2SO4. Remarkably, this catalyst displays remarkable stability in the water splitting process. It is shown that the introduction of Pt could augment oxygen vacancies and enhance catalytic activity significantly. The synergy between Pt and Ru further contributes to the improved performance. Additionally, we have observed that acidic seawater holds distinct advantages for acidic water splitting, along with a thorough exploration of the relationship between Cl− concentration and catalytic performance. This study not only provides a strategy to improve the catalytic activity and stability of ruthenium-based catalysts for water splitting in acidic environments, but also unveils the promoting effect of Cl− on the catalytic activity in acidic water splitting.