Fangwen Peng, Zhen Luo*, Yingjin Ma*, Haibo Ma*
Chin. J. Struct. Chem., 2024, 43: 100273. DOI: 10.1016/j.cjsc.2024.100273
May 15, 2024
ABSTRACT
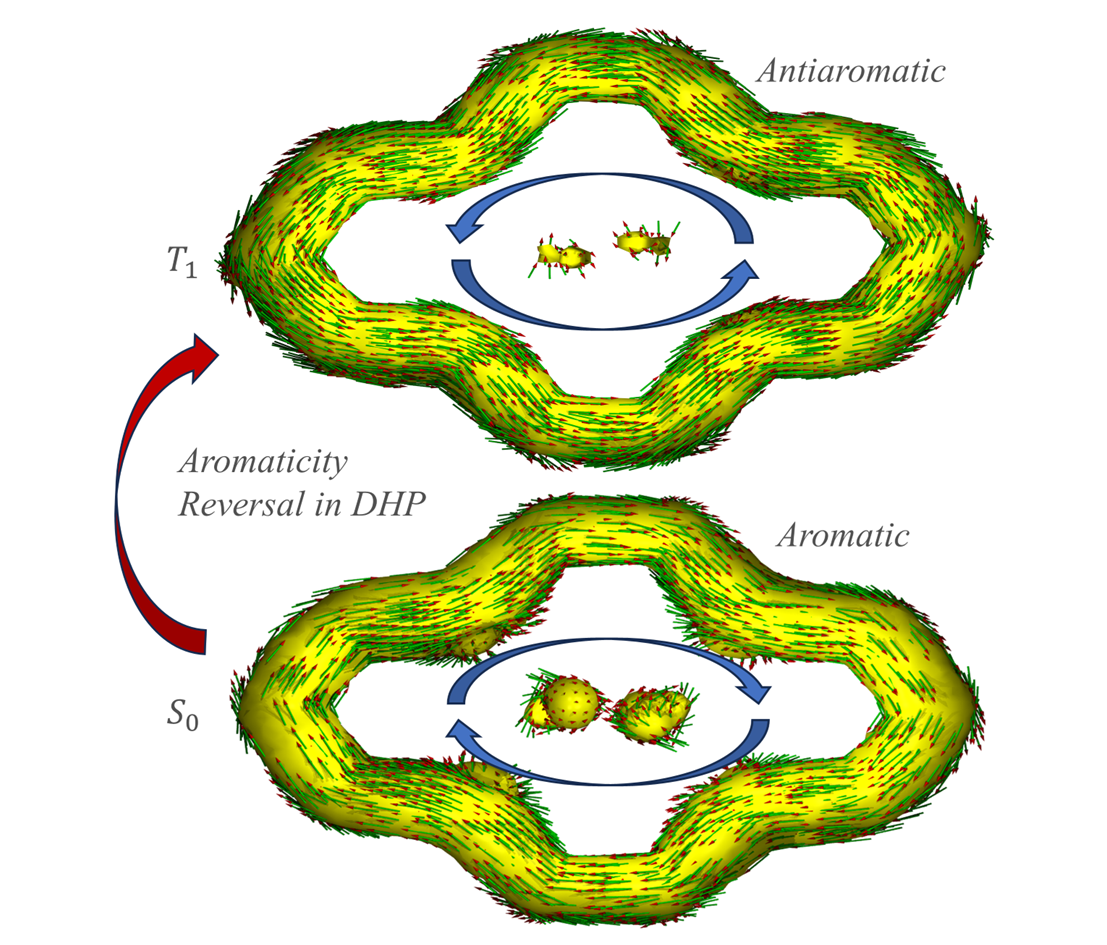
In conclusion, we apply four aromaticity indices, including NICS, ACID-π, EDDB and HOMA methods, to evaluate (anti-)aromaticity of DHP systems for the S0 and T1/S1 states. All the four indices agree that DHP systems experience aromaticity reversal when excited to T1 state. More importantly, the MCSCF-GIAO method demonstrates that Baird's rule holds for both T1 and S1 states of such systems from a theoretical point of view. Furthermore, we find that t-Bu-DHP would undergo aro[1]matic reduction in S0 states when conjugated moieties like acetylenyl are introduced as its side-chain groups according to corresponding values. These findings may shed light on the design and development of materials concerning photochemical reactions. In addition, their radical character is also studied, and we discover that t-Bu-DHP is more likely to form radicaloids during excitation when an acetylenyl group is introduced.
It should be noted that, however, quantitatively reliable NICS for excited-state acety-t-Bu-DHP is not guaranteed by the above computation, since MCSCF-GIAO can't afford the description of full valence π space in this system, which would be indispensable for excited states. When involving large active space, more accurate NICS calculation has to overcome the exponential growth of the FCI expansion. Therefore, DMRG's marriage to MCSCF-GIAO is supposed to be put on the agenda, and our laboratory is striving to be their matchmaker.