Insight into stable, concentrated radicals from sulfur-functionalized alkyne-rich crystalline frameworks and application in solar-to-vapor conversion
Jian-Rong Li, Jieying Hu , Lai-Hon Chung, Jilong Zhou, Parijat Borah, Zhiqing Lin, Yuan-Hui Zhong, Hua-Qun Zhou, Xianghua Yang, Zhengtao Xu*, Jun He*
Submit a Manuscript
Mengjun Zhao, Yuhao Guo, Na Li*, Tingjiang Yan*
Chin. J. Struct. Chem., 2024, 43: 100348. DOI: 10.1016/j.cjsc.2024.100348
August 15, 2024
Iron oxides; Photocatalysis; CO2 hydrogenation; Structural evolution
ABSTRACT
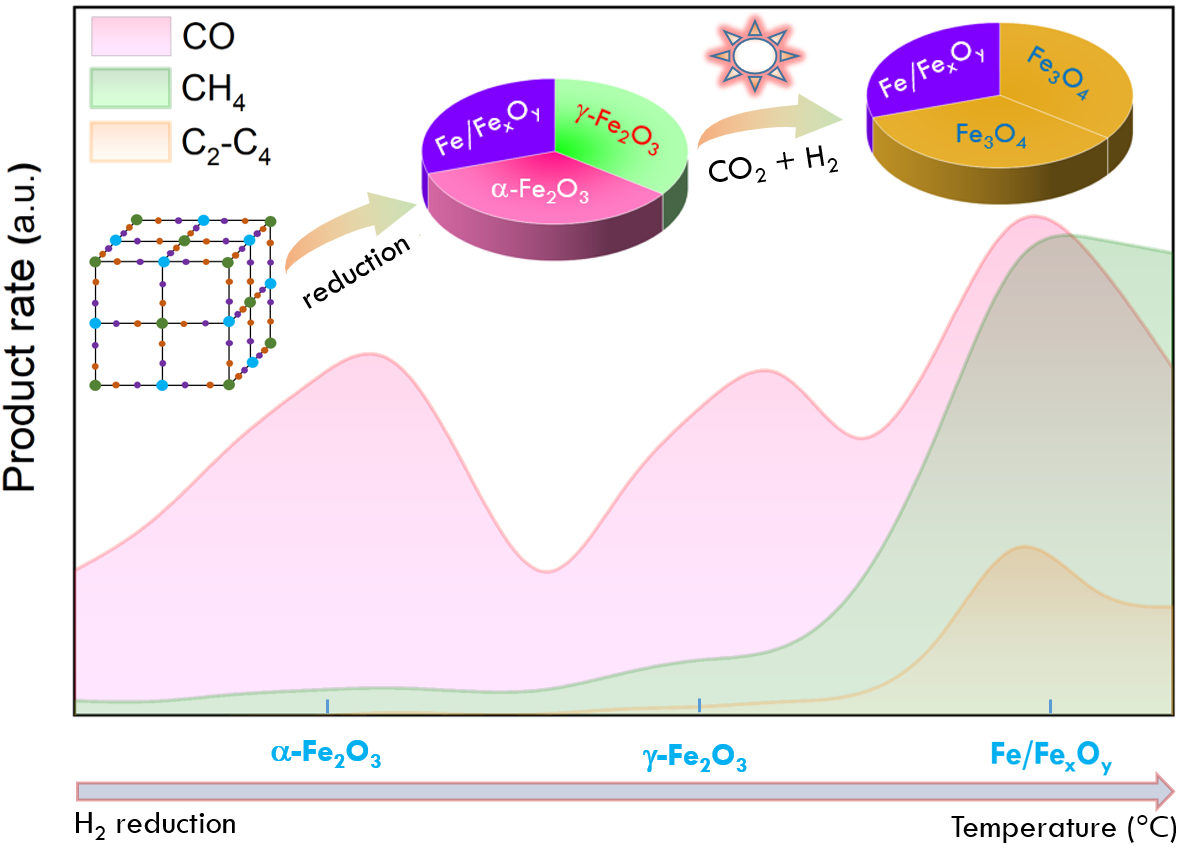
Photocatalytic CO2 hydrogenation reactions can produce high-value-added chemicals for industry, solving the environmental problems caused by excessive CO2 emissions. Iron oxides are commonly used in photocatalytic reactions due to their various structures and suitable band gaps. Nevertheless, the structural evolution and real active components during photocatalytic CO2 hydrogenation reaction are rarely studied. Herein, a variety of iron oxides including α-Fe2O3, γ-Fe2O3, Fe3O4 and FeO were derived from Prussian blue precursors to investigate the CO2 hydrogenation performance, structural evolution and active components. Especially, the typical α- and γ-Fe2O3 are converted to Fe3O4 during the reaction, while Fe/FexOy remains structurally stable. Meanwhile, it is confirmed that Fe3O4 is the main active component for CO production and the formation of hydrocarbons (CH4 and C2–C4) are highly dependent on the Fe/FexOy heterojunctions. The optimal yields of CO, CH4 and C2–C4 hydrocarbons over the best catalyst (FeFe-550) can achieve 4 mmol g−1 h−1, 350 μmol g−1 h−1 and 150 μmol g−1 h−1, respectively due to their suitable metal/oxide component distribution. This work examines the structural evolution of different iron oxide catalysts in the photocatalytic CO2 hydrogenation reaction, identifies the active components as well as reveals the relationship between components and the products, and offers valuable insights into the efficient utilization of CO2.