Cover Picture
Nano-flowers FeS/MoS2 composites as a peroxymonosulfate activator for efficient p-chlorophenol degradation
Xian-Rui Meng, Qian Chen, Mei-Feng Wu, Qiang Wu, Su-Qin Wang*, Li-Ping Jin, Fan Zhou, Ren-Li Ma*, Jian-Ping Zou* Submit a Manuscript
Nano-flowers FeS/MoS2 composites as a peroxymonosulfate activator for efficient p-chlorophenol degradation
Xian-Rui Meng, Qian Chen, Mei-Feng Wu, Qiang Wu, Su-Qin Wang*, Li-Ping Jin, Fan Zhou, Ren-Li Ma*, Jian-Ping Zou* Submit a Manuscript
Nano-flowers FeS/MoS2 composites as a peroxymonosulfate activator for efficient p-chlorophenol degradation
Xian-Rui Meng, Qian Chen, Mei-Feng Wu, Qiang Wu, Su-Qin Wang*, Li-Ping Jin, Fan Zhou, Ren-Li Ma*, Jian-Ping Zou*
Chin. J. Struct. Chem., 2025, 44(3), 100543. DOI: 10.1016/j.cjsc.2025.100543
FeS/MoS2 nano-flowers; Peroxymonosulfate activation; p-chlorophenol degradation; Sulfur vacancies; Advanced oxidation processes
ABSTRACT
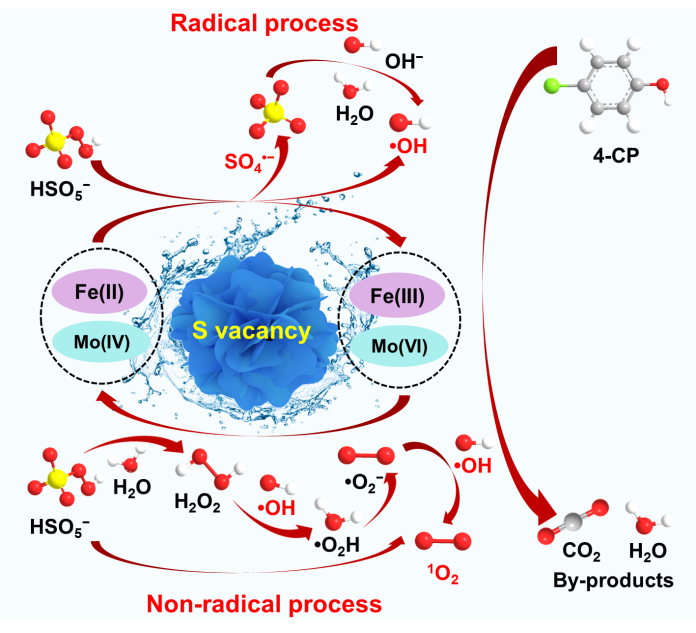
The degradation of organic pollutants in water is a critical environmental challenge. The iron-doped MoS2 catalysts have demonstrated potential in activating peroxymonosulfate (PMS) for environmental remediation, but they face challenges such as poor conductivity, limited electron transfer efficiency, and a scarcity of active sites. To address these issues, we successfully synthesized a nano-flowers FeS/MoS2 composite derived from polyoxometalates (NH4)3[Fe(III)Mo6O24H6]·6H2O (denoted as FeMo6) as the bimetallic precursors. This synthesis strategy enhances the interaction between FeS and MoS2, thereby facilitating electron transfer. Notably, the introduction of sulfur vacancies in FeS/MoS2 exposes additional Mo4+ active sites, facilitating the redox cycle of Fe2+/Fe3+ and accelerating the regeneration of Fe2+, which in turn enhances PMS activation. Therefore, a catalytic oxidation system of FeS/MoS2/PMS is presented that primarily relies on SO4•- and •OH, with 1O2 as a supplementary oxidant. This system exhibits exceptional degradation efficiency for p-chlorophenol (4-CP), achieving 100% degradation within 10 minutes over a wide pH range of 2.4 to 8.4. The robust performance and wide applicability of FeS/MoS2 catalyst make it a promising candidate in advanced oxidation processes (AOPs) for environmental remediation.