Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif
SUN Guo-Xiang, WANG Qiao, MIN Li-Jing, HAN Liang and LIU Xing-Hai*
Chin. J. Struct. Chem. 2022, 41, 2202114-2202122 DOI: 10.14102/j.cnki.0254-5861.2011-3249
February 15, 2022
acyl urea compounds, crystal structure, synthesis, antifungal activity, docking
ABSTRACT
Eighteen
new acyl urea derivatives containing 2-chloronicotine moiety were synthesized
using 2-chloronicotinic acid as starting material via 4 steps conveniently.
These 2-chloronicotine acyl urea structures were confirmed by 1H
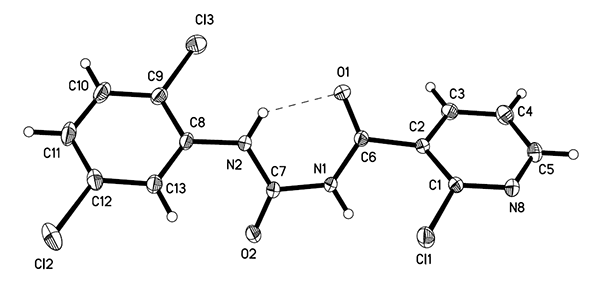
NMR, 13C NMR and HRMS. Compound 4r was further confirmed by X-ray diffraction. It crystallizes in the
orthorhombic system, space group Pbca with a = 7.2960(3), b =
14.8546(6), c =
25.2840(11) Å, V =
2740.3(2) Å3, Z = 8, the final R = 0.0442 and wR = 0.1033 for 4028 observed reflections with I >
2σ(I). The
antifungal activity results demonstrate that some of these compounds possessed
good activity against B. cinerea, G. zeae, P. piricola, and P. Capsici at 50 ppm. Further molecular docking results indicated that the key group is urea
bridge and pyridine ring.