Molecular Design and Property Prediction of High Density 4-Nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate Derivatives as the Potential High Energy Explosives
YANG Jing*, PANG Yu, LI Min-Xian, YANG Ge-Fei, JIA Jing-Xian, MENG Xiang-Jun, LIU Li-Hua, YANG Xiao-Chun and GAO Xiao-Zhen
Chin. J. Struct. Chem. 2022, 41, 2202123-2202131 DOI: 10.14102/j.cnki.0254-5861.2011-3256
February 15, 2022
4-nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate, energetic materials, density functional theory, explosive
ABSTRACT
To search for potential energetic materials with large energy density
and acceptable thermodyna- mics and kinetics stability, twelve derivatives of
4-nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate (named A~L) are designed and
analyzed by using density functional theory (DFT) calculations at the B3LYP/6-311G**
level of theory. The molecular heats of formation (HOF), electronic structures,
impact sensitivity (H50), oxygen balance (OB) and density (ρ) are investigated by isodesmic reaction method and physicochemical
formulas. Furthermore, the detonation velocity (D) and detonation
pressure (P) are calculated to study the detonation performance by Kamlet-Jacobs
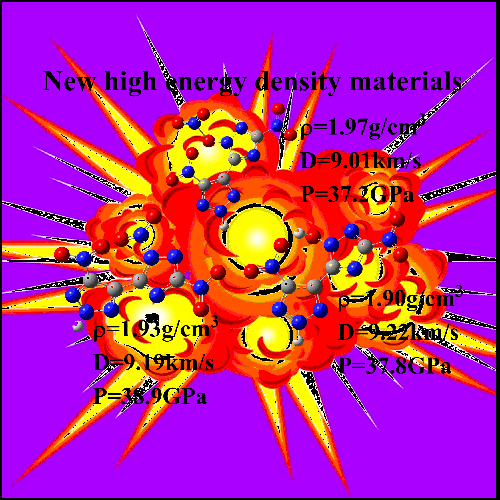
(K-J) equation. These results show that new molecule J (H50 =
36.9 cm, ρ = 1.90 g/cm3, Q = 1912.46
cal/g, P = 37.82 GPa, D = 9.22 km/s, OB = 0.00), compound A (H50 = 27.9 cm, ρ = 1.93 g/cm3, Q = 1612.93 cal/g, P = 38.90 GPa, D = 9.19 km/s) and
compound H (H50 = 37.3 cm, ρ = 1.97 g/cm3, Q = 1505.06 cal/g, P =
37.20 GPa, D = 9.01 km/s) present promising effects that are far better
RDX and HMX as the high energy density materials. Our calculations can provide
useful information for the molecular synthesis of novel high energy density
materials.