Novel 2,4-Diarylaminopyrimidine Derivatives Containing Pyridine Moiety: Design, Synthesis, Crystal Structure and Biological Evaluation
LIU Ju, WU Shuang, WANG Huan, DU Si-Yuan, LI Zhen, SHEN Ji-Wei, CHEN Ye* and DING Shi*
Chin. J. Struct. Chem. 2022, 41, 2202132-2202140 DOI: 10.14102/j.cnki.0254-5861.2011-3283
February 15, 2022
pyrimidine, pyridine, synthesis, X-ray diffraction, antitumor activity
ABSTRACT
A series of 2,4-diarylaminopyrimidine derivatives containing pyridine
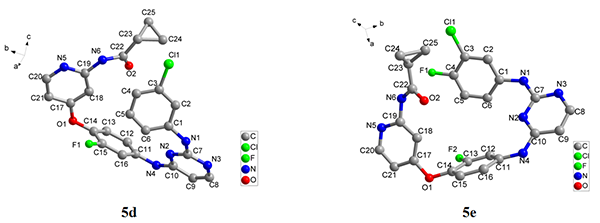
structure were designed and synthesized. The crystal structures of compounds 5d and 5e were obtained from X-ray diffraction. The crystal structure of 5d (C25H20ClFN6O2)
belongs to the monoclinic system,
space group P21/c with a = 11.0500(10), b = 18.3045(17), c = 13.5646(9) Å and β = 122.806(5)°. 5e (C25H19ClF2N6O2)
is of monoclinic system, space group P21/c with a = 10.9998(18), b = 18.517(3), c = 13.6355(16)
Å and β = 123.315(9)°. The bioassay results showed
all of the target compounds exhibited potential antiproliferative activities
against MKN-45, HT-29, A549, K562 and GIST882 cell lines. Among them, compounds
5a , 5c and 5e exhibited remarkable
inhibitory activities against GIST882, K562 and A549 cell lines with IC50 values
of 0.68, 0.38 and 0.60 μM, respectively, which were comparable to
that of the positive control foretinib.