Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives
SUN Yue, YANG Zi-Hui and GU Wen*
Chin. J. Struct. Chem. 2022, 41, 2202098-2202104 DOI: 10.14102/j.cnki.0254-5861.2011-3232
February 15, 2022
furan-1,3,4-oxadiazole carboxamide, synthesis, crystal structure, antifungal activity
ABSTRACT
A series of novel
furan-1,3,4-oxadiazole carboxamide derivatives (5a~5e)
were designed, synthesized and characterized by spectroscopic methods including
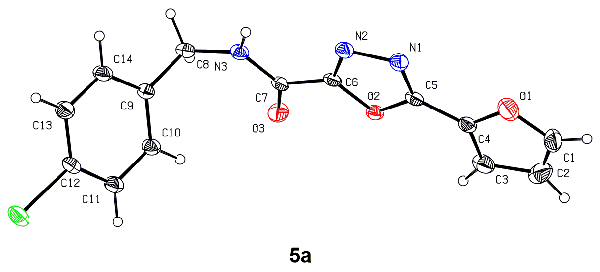
HR-MS, 1H- and 13C-NMR. The crystal structure of compound 5a was determined by single-crystal X-ray diffraction. The compound
crystallizes in the triclinic system, space group P with a = 4.7261(5), b = 10.4672(11), c =
14.5886(13) Å, α = 106.081(4)°, β = 91.043(3)°, γ = 99.456(4)°, Z = 2, V = 682.48(12) Å3, Mr = 348.16, Dc = 1.694 Mg/m3, S = 1.008, m = 3.025 mm-1, F(000) = 348, the final R = 0.0775 and wR = 0.2080 for 2774 observed reflections (I > 2σ(I)). There are two
kinds of hydrogen bonds (N(3)–H(3A)×××N(2) and C(8)–H(8A)×××O(3)) present in its crystal structure. The preliminary antifungal assay showed
that compounds 5b and 5c exhibited significant antifungal activities against several plant
pathogenic fungi.