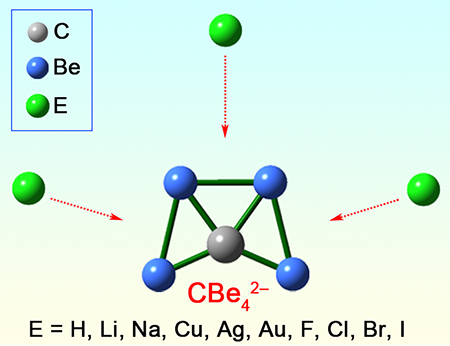
Planar Tetracoordinate Carbon in 6s + 2p Double Aromatic CBe42– Derivatives
JIN Bo, BIAN Jian-Hong, ZHAO Xue-Feng, YUAN Cai-Xia, GUO Jin-Chang and WU Yan-Bo*
Chin. J. Struct. Chem. 2022, 41, 2203218-2203226 DOI: 10.14102/j.cnki.0254-5861.2011-3332
March 15, 2022
planar hypercoordinate carbon, aromaticity, beryllium, DFT calculations, global energy minimum
ABSTRACT
As a typical electron
deficient element, beryllium is potentially suitable for designing the species
with novel non-classical planar hypercoordinate carbon due to high preference
for the planar structures by small beryllium-containing
clusters. In particular, the CBe54– cluster with a planar
pentacoordinate carbon (ppC) had been proved by many previous studies to be an
excellent template structure for the systematic design of ppC species through
attaching various monovalent atoms on the bridging position of Be–Be edges. In this work, based on the analysis
and extension on our recently reported CBe4Mnn–2 (M = Li, Au, n = 1~3) species, we propose that
ptC cluster CBe42– is similar to CBe54– in that it can also be employed as a
template structure to systematically design the ptC species through binding
various monovalent atoms on the bridging position of Be–Be edges. Our extensive screening suggests that the feasible bridging
atoms (E) can be found in group 1 (H, Li, Na), group 11 (Cu, Ag, Au), and group
17 (F, Cl, Br, I) elements, leading to total thirty eligible ptC species with
CBe4 core moiety (CBe4Enn–2). The ptC atoms in these
species are involved into three delocalized s bonds and a delocalized p bond, thereby not only obeying the octet rule, but
also possessing novel 6s +2p double aromaticity, which
significantly stabilizes the ptC arrangement. In addition, the attached
bridging atoms can stabilize the CBe4 core ptC moiety by replacing
the highly diffused Be–Be
two-center two-electron bonds with the much less diffused Be–E two-center
two-electron bonds or Be–E–Be
three-center two-electron bonds, as reflected by the increasing HOMO-LUMO gaps
when the number of bridging atoms increases. Remarkably, the stochastic search
algorithm in combination with high level CCSD(T) calculations revealed that
twenty-six of the thirty-one ptC species (including previously reported six species)
were global energy minima on their corresponding potential energy surfaces, in
which twenty-five of them were also confirmed to be dynamically viable. They
are suitable for the generation and characterization in gas phase experiments
and followed spectroscopic studies.