Theoretical Study of Non-heme Mn(V)-oxo Complexes: Formation Mechanism, Electronic Nature, and Hydroxylation Reactivity
WEN Zi-Hao, LI Shuang and ZHANG Min-Yi*
Chin. J. Struct. Chem. 2021, 40, 1557-1566 DOI: 10.14102/j.cnki.0254-5861.2011-3209
December 15, 2021
C–H bond activation, non-heme manganese complex, homogeneous catalysis, density functional theory calculations
ABSTRACT
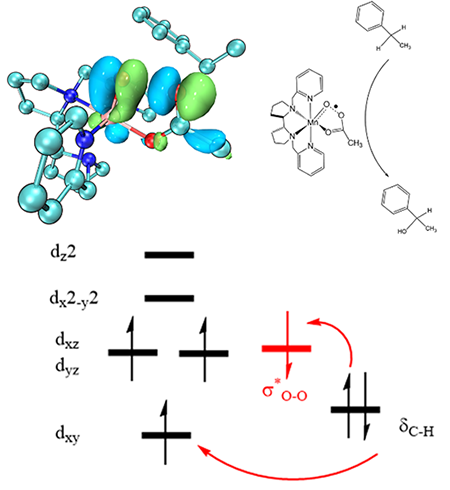
Many non-heme manganese complexes exhibit high reactivity and enantioselectivity for the activation of C–H bonds. Recently, Mn(PDP) complexes (PDP = N,N΄-bis(pyridine-2-ylmethyl)-2,2΄-bipyrrolidine) have been reported to activate C–H bonds selectively in the presence of carboxylic acids. In this study, we performed density functional theory calculations to investigate the formation and hydroxylation mechanisms of Mn(PDP) complexes. Our calculation results showed that Mn(III)(PDP) complexes react with H2O2 and carboxylic acid to form Mn(V)-oxo oxidation intermediate. The main oxidation intermediate, [(PDP)Mn(IV)(O···OC(O)CH3)2-·]2+, was found to have the characteristics of S(Mn) = 3/2 manganese(IV) center antiferromagnetically coupled to a σ*O–O radical, where the O–O bond is not completely broken. Furthermore, [(PDP)Mn(IV)(O···OC(O)CH3)2-·]2+ was shown to have two single electron-accepting orbitals (α Mn-dxy and β σ*O–O) that can simultaneously interact with a doubly occupied electron-donating orbital (σC–H) of substrate. Therefore, [(PDP)Mn(IV)(O···OC(O)CH3)2-·]2+ species can act as a two-electron oxidant for the C−H bond functionalization. As a result, the C−H bond hydroxylation by [(PDP)Mn(IV)(O···OC(O)CH3)2-·]2+ species was a single step. Following the H-abstraction with a low barrier of 4.5 kcal/mol, hydroxyl group would immediately rebound to the radical carbon without barrier. These results provide new insights toward the further development of non-heme manganese catalysts.