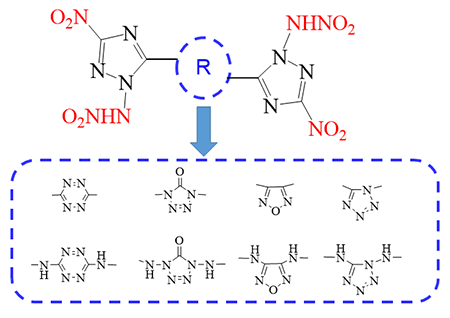
Bridged Effects of Various Heterocyclic Linkages in Bis-1,2,4-triazoles
WU Jin-Ting*, XU Jin, LI Hong-Bo* and ZHANG Jian-Guo
Chin. J. Struct. Chem. 2021, 40, 1433-1438 DOI: 10.14102/j.cnki.0254-5861.2011-3181
December 15, 2021
density functional theory, effect of heterocyclic linkage, energetic material, detonation performance
ABSTRACT
There are numerous studies on nitrogen-rich heterocycles explosive
design and synthesis due to their good detonation activity. A series of bistriazoles with different heterocyclic linkages
were designed and calculated by density functional theory (DFT) b3lyp/6-311+G*
method. The structure, detonation properties and stability of the energetic
compounds have been investigated. According to the results from heats of
formation (HOFs), the HOF values of bistriazole with heterocycle
linkage (M1~M4) are higher than those
of the corresponding diamino-heterocycle bridged ones (M5~M8). By analyzing the bond
dissociation energy (BDE), -NH- is not conducive to increase the stability of
the derivatives. In terms of detonation performances and stability of bistriazole
derivatives, the combination of furazan or tetrazole linkages with
bis-triazoles may be considered as potential candidates for energetic
materials.