Theoretical Study of Iron Porphyrin Imine Specie of P411 Enzyme: Electronic Structure and Regioselectivity of C(sp3)-H Primary Amination
LI Shuang, WEN Zi-Hao and ZHANG Min-Yi*
Chin. J. Struct. Chem. 2021, 40, 1411-1422 DOI: 10.14102/j.cnki.0254-5861.2011-3191
December 15, 2021
DFT, cytochrome P411 enzyme, C–H bond activation, enzyme catalysis
ABSTRACT
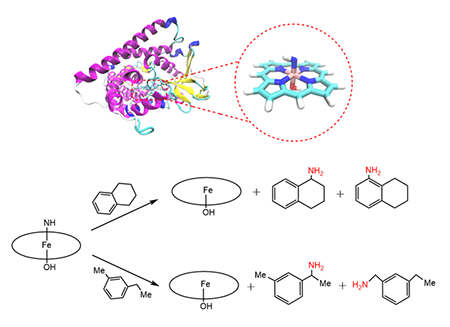
The cytochrome P411 enzyme is a
variant of cytochrome P450BM3 from Bacillus megaterium whose active site is an iron porphyrin imine ([Fe(Por)(NH)]-)
specie. This specie has been reported to successfully promote the primary amination of benzylic and allylic C(sp3)−H
bonds. We employed density functional theory to study the electronic structure
of the active site of P411 enzyme and the
primary amination of C−H bond reaction that it catalyzes. The calculated spin densities and
orbital values indicate the existence of resonance
in this specie; namely, [(por)(–OH)FeIV–N2-–H]- ↔ [(por)(–OH)FeIII–N•-–H]-.
The amination of C(sp3)−H bonds consists of
two main reaction steps: hydrogen-atom abstraction and
radical recombination, and the former is demonstrated to be the
rate-determining step. Furthermore, we studied the regioselectivity of the
amination of primary and secondary C(sp3)−H bonds. Our
calculations indicated that the secondary C(sp3)−H bonds of the substrate would be more favored for the activation
by P411 enzyme. These results provide valuable information for understanding
the properties and selectivity of C−H/C−N bond-activation reactions catalyzed
by the P411 enzyme or other similar enzymes.