Synthesis, Crystal Structure and Its Application in Living
Cells Imaging of Benz(c)acridine-1,2,3-triazole Derivatives
YAN Zhen-Shuo, KANG Yan-Hui, CHEN Yang-Ling and HUO Li-Ni*
Chin. J. Struct. Chem. 2021, 40, 1309-1316 DOI: 10.14102/j.cnki.0254-5861.2011-3159
October 15, 2021
benz(c)acridine-1,2,3-triazole, crystal structure, fluorescent probe, iron ions
ABSTRACT
A highly selective
iron ions fluorescent probe based on the benz(c)acridine-1,2,3-triazole
derivatives was produced by multi-step reactions. 1-(7-Benz[c]acridinyl)-4-(4-methylphenyl)-1,2,3-triazole
(4a), C26H18N4, was
structurally determined by single-crystal X-ray diffraction. It crystallizes in
the trigonal system, space group R-3
with a = 36.230(10), b = 36.230(10), c = 7.993(3) Å, β = 90°, V = 9086(6) Å3, Z = 18, Dc = 1.271 g/cm3, F(000) = 3636, μ = 0.602 mm-1,
the final R = 0.0865 and wR = 0.1619 for 3951 observed reflections (I > 2σ(I)). X-ray analysis
indicated that all four rings of benz(c)acridine are in the same plane, and the
1,2,3-triazole ring and the corresponding linked benzene (C(1)–C(6)–C(7)–C(8)–C(9)–N(1)) are approximately
perpendicular with a dihedral angle of 106.5°. The crystal
packing of the compound was stabilized by two weak interactions between
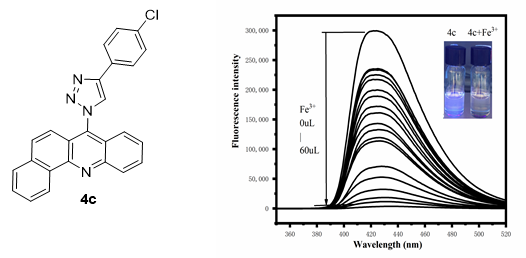
C(11)–H(10)···N(4) and C(18)–H(18)···N(1). In fluorescence spectra studies, compound 4c was exhibited good selectivity and sensitivity
towards iron ions in DMSO: mops buffer solution. Furthermore, 4c was successfully used for imaging iron ions in
living HeLa cervical cancer cells.