Synthesis, Structure and Characterization
of a New Complex: [Mn(C6H12N4)2(H2O)4][Mn(H2O)6][SO4]2·6H2O
LI Deng-Peng, XU Zhi-Huang, YE Li-Wang, CAO Teng-Fei and ZHUANG Xin-Xin*
Chin. J. Struct. Chem. 2021, 40, 1169-1176 DOI: 10.14102/j.cnki.0254-5861.2011-3119
September 15, 2021
hexamethylenetetramine, crystal structure, thermal stability, susceptibility
ABSTRACT
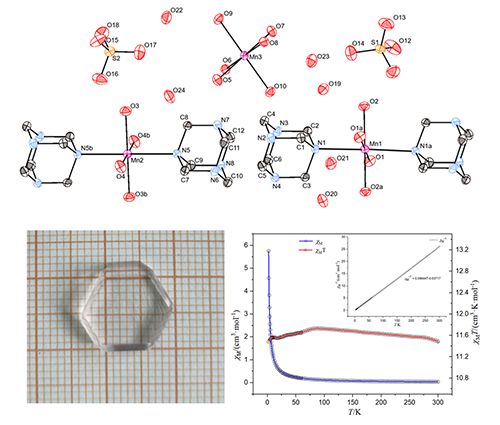
A novel complex, [Mn(C6H12N4)2(H2O)4][Mn(H2O)6][SO4]2·6H2O,
was synthesized and hexagonal single crystals with centimeter-scale
sizes were obtained by the method of solvent evaporation. It was characterized
by elemental analysis, infrared spectrum, thermogravimetric analysis and X-ray
single-crystal diffraction. The complex belongs to triclinic crystal system,
space group P with a = 9.3390(8), b = 13.3520(13), c = 16.3207(13) Å, α = 100.7160(3)°, β = 90.1020(10)°, γ = 109.9490(5)°, V = 1874.9(3) Å3, Z = 2, Dc = 1.542
g/cm3, Mr = 870.64, μ = 0.876
mm-1, T = 293(2) K, F(000) = 916 and S = 0.990. The crystal structure
determination displayed a distorted octahedral geometry around the manganese atom,
which is bound to two nitrogen atoms from hexamethylenetetra- mine, acting as
monodentate ligands, and to four aqua ligands. Variable-temperature magnetic
measurements of the complex indicate the presence of weak antiferromagnetic interaction between manganese centers.