Syntheses, Crystal Structures and Different Magnetic Behaviors of Three Cyanide-bridged FeII-MII (M = Fe, Co and Mn) Complexes
HUANG Ying-Ying, XU Qing-Dou, HU Sheng-Min, WU Xin-Tao and SHENG Tian-Lu*
Chin. J. Struct. Chem. 2021, 40, 1161-1168 DOI: 10.14102/j.cnki.0254-5861.2011-3123
September 15, 2021
cyanide-bridged, magnetic property, spin crossover
ABSTRACT
Three
dinuclear cyanide-bridged complexes [FeII(PY5OMe2)CNMII(PY5OMe2)](OTf)3 (M = Fe 1, M = Co 2, M = Mn 3) (PY5OMe2 = 2,6-bis-((2-pyridyl)methoxymethane)pyridine OTf
= CF3SO3-) have
been synthesized and characterized. Single-crystal diffraction analyses show
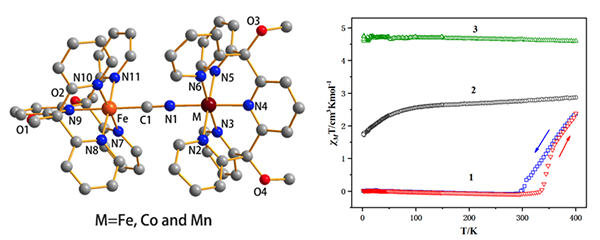
these three dinuclear compounds are very similar in structure. The measured ν(CN) results for compounds 1~3 suggest that Mn2+ is electron-poorer than Fe2+ and Co2+. Meanwhile, the
temperature dependence of magnetic susceptibilities of complexes 1~3 reveals that in these three complexes, all the cyanide-carbon coordinated Fe(II) is low-spin, and Co(II) for 2 and
Mn(II) for 3 are both in a high-spin
state through 2~400 K but the cyanide-nitrogen
coordinated Fe(II) for complex 1 exhibits spin crossover (SCO) behavior over 300 K and a hysteresis of 36 K in both cooling and heating modes.