High Rate Performance of Aqueous Magnesium-iron-ion Batteries Based on Fe2O3@GH as the Anode
SHAO Shuang-Xi, CANG Rui-Bai, YE Ke, GAO Yin-Yi, ZHU Kai, YAN Jun, WANG Gui-Ling and CAO Dian-Xue*
Chin. J. Struct. Chem. 2021, 40, 908-918 DOI: 10.14102/j.cnki.0254-5861.2011-3063
July 15, 2021
aqueous battery, Mg ion, Fe2O3/graphene hydrogels, anode material, FeSO4
ABSTRACT
Aqueous Mg-ion
batteries (MIBs) are safe, non-toxic and low-cost. Magnesium has a high theoretical specific
capacity with its ion radius close to that of lithium. Therefore, aqueous
magnesium ion batteries have great research advantages in green energy. To
acquire the best electrode materials for aqueous magnesium ion batteries, it is
necessary for the structural design in material. Fe2O3 is
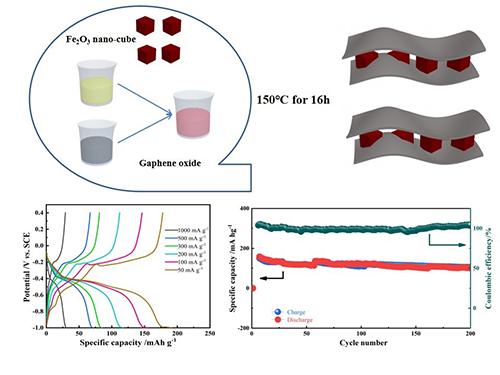
an anode material commonly used in Li-ion battery. However, the nano-cube Fe2O3 combined with graphene hydrogels (GH) can be successfully prepared and employed
as an anode, which is seldom researched in the aqueous batteries system. The Fe2O3/GH
is used as anode in the dual MgSO4 + FeSO4 aqueous electrolyte, avoiding the irreversible deintercalation of magnesium
ions. In addition, the Fe element in anode material can form the Fe3+/Fe2+ and Fe2+/Fe3+ redox pairs in the MgSO4 +
FeSO4 electrolyte. Thus, the reversible insertion/(de)insertion of
magnesium and iron ions into/from the host anode material can be simultaneously
achieved. After the initial charge, the anodic structure is changed to be more
stable, avoiding the formation of MgO. The Fe2O3/GH
demonstrates high rate properties and reversible capacities of 198, 151, 121,
80, 75 and 27 mAh g−1 at 50, 100, 200, 300, 500 and 1000 mA g−1 correspondingly.