Haloplumbate Nanochains Templated by Quaternary Phosphorus: Good Water Stabilities, Thermochromic Luminescence and Photocurrent Responses
CUI Jian-Hao, ZHANG Wen-Ting, ZHENG Hui-Dong* and LI Hao-Hong*
Chin. J. Struct. Chem. 2021, 40, 775-784 DOI: 10.14102/j.cnki.0254-5861.2011-3009
June 15, 2021
organic-inorganic hybrid, haloplumbate, quaternary phosphorus, photoluminescence, photocurrent response
ABSTRACT
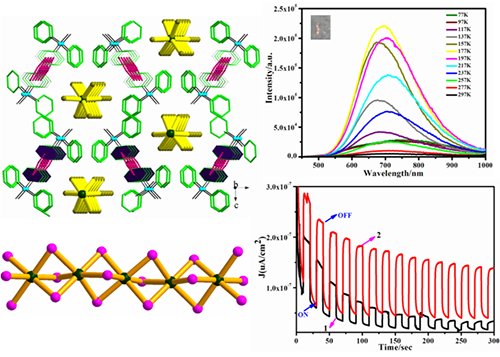
Two triphenylmethylphosphonium/haloplumbate hybrids, i.e., [(PPh3Me)2(Pb2I6)∙CH3CN]n (1) and [(PPh3Me)(PbBr3)]n (2), have been prepared, in which the (PbX3)nn- nanochains built from face-sharing PbX6 octahedra are surrounded by organic templates to assemble
the core-shell quantum well. Besides, C−H···π interactions among Ph3PMe+ cations can also be detected, which give rise to the 2-D organic layer of 1 and
1-D chain for 2. The good water stabilities could be induced by
the strong C−H···π interactions, which can deter the hydrolysis
reaction. The energy band gaps of this work mainly derive from
the charge transfer of organic components, but their luminescence stems from the inorganic (PbX3)nn- nanochains with co-existence of free excitons and self-trapped excitons.
At temperature lower than 117 K, strong quantum confinement will rule out the free
excitons, and self-trapped excitons will dominate, resulting in red-shift
luminescence. Moreover, effective and
repeatable photocurrent responses can be found in these hybrids.