Synthesis, Crystal Structure and Property Studies on a Bipyridine Adduct of Nickel Xanthogenate
LIU Xiao-Jing, LIU E, LI Zhuang-Yu and JIAN Fang-Fang*
Chin. J. Struct. Chem. 2021, 40, 588-594 DOI: 10.14102/j.cnki.0254-5861.2011-2986
May 15, 2021
DBPMF, coordination polymer, xantghogenate, crystal structure, fluorescence
ABSTRACT
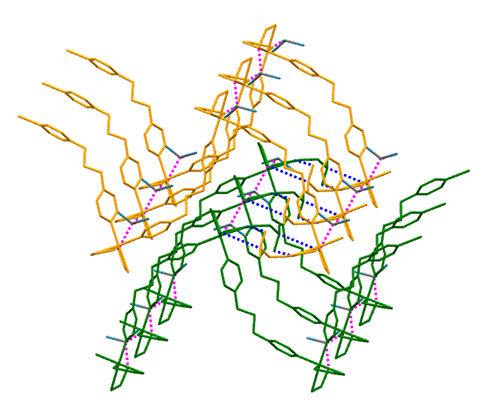
A new coordination polymer containing mixed
ligands, namely [Ni(S2COiBu)2(DBPMF)·(CHCl3)]∞ (DBPMF = 2,7-dibromo-9,9-(4-pyridylmethyl)fluorine),
has been synthesized and structurally characterized by elemental analysis and
single-crystal X-ray diffractions. X-ray structural analysis reveals that each
Ni atom is coordinated by two isobutyl xanthogenate molecules and two nitrogen
atoms from two DBPMF ligands to form octahedral coordination geometry. DBPMF
acts as a bridge to form a coordination polymer. Fluorescence property of this
coordination polymer displays a broad emission band at λ = 413 nm. Thermal analysis shows that the solvent molecule CHCl3 can be stabilized in the crystal structure until 140 °C.