Theoretical Analysis of the Mechanism of Cationic
Pd(II)-catalyzed Fujiwara-Moritani Reaction
REN Ying*, WANG Tao, ZHANG Ting-Ting, JIA Jian-Feng and WU Hai-Shun
Chin. J. Struct. Chem. 2021, 40, 576-587 DOI: 10.14102/j.cnki.0254-5861.2011-3077
May 15, 2021
C−H bond activation, DFT studies, Pd(II) catalyst, reaction mechanism
ABSTRACT
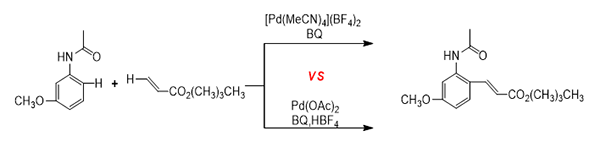
A systematic theoretical
investigation has been studied on Fujiwara-Moritani reaction between
3-methoxyacetanilide with n-butyl
acrylate by means of density functional theory (DFT) calculations when two
types of Pd(II) catalysts are employed. In [Pd(MeCN)4](BF4)2 catalytic cycle, a 1,4-benzoquinone(BQ)-induced C−H activation of trans-(MeCN)2Pd(BQ)2+ with 3-methoxyacetanilide occurs as the first step to give DC-4MeCN,
facilitating the insertion of n-butyl
acrylate and β-hydride elimination,
followed by recycling of catalyst through hydrogen abstraction of monocationic
BQ fragment. In Pd(OAc)2 catalytic cycle, it is proposed that the
most favored reaction pathway should proceed in dicationic mechanism involving
a BQ-assisted hydrogen transfer for C−H activation by Pd active catalyst (HOAc)2Pd(BQ)2+ to generate DC-4HOAc, promoting acrylate insertion and β-hydride elimination, followed by the regeneration
of catalyst to give the final product. The calculations indicate that the
rate-determining step in [Pd(MeCN)4](BF4)2 catalytic system is the acrylate insertion, while it is the regeneration of
catalyst in the Pd(OAc)2 catalytic system. In particular, the roles
of BQ and ligand effects have also been investigated.