Surface Oxidation of Single-walled-carbon-nanotubes with Enhanced Oxygen Electroreduction Activity and Selectivity
CUI Ya-Qi, XU Jiao-Xing*, WANG Mei-Lin and GUAN Lun-Hui*
Chin. J. Struct. Chem. 2021, 40, 533-539 DOI: 10.14102/j.cnki.0254-5861.2011-3157
May 15, 2021
electrocatalysts, hydrogen peroxide production, oxygen reduction reaction
ABSTRACT
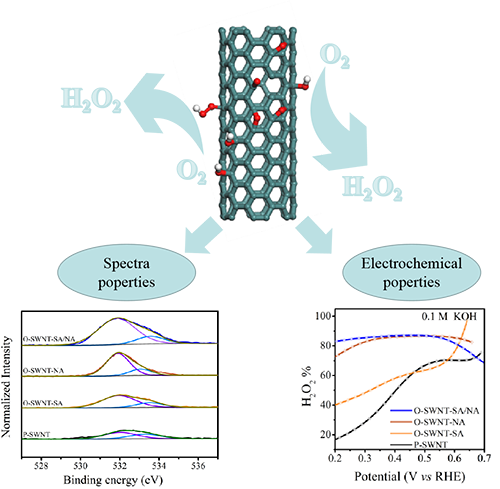
Electrochemical oxygen reduction reaction (ORR) with 2-electron
process is an alternative for decentralized H2O2 production, but it remains
high challenging to develop highly active and selective catalysts for this process.
In this work, we present a selective and efficient nonprecious electrocatalyst,
prepared through an easily scalable mild oxidation of single-walled carbon
nanotubes (SWNTs) with different oxidative acids including sulfur acid, nitride
acid and mixed sulfuric/nitric acids, respectively. The high-degree oxidized
SWNTs treated by mixed acids exhibit the highest activity and selectivity of
electroreduction of oxygen to synthesize H2O2 at low
overpotential in alkaline and neutral media. Spectroscopic characterizations suggested
that the C–O is vital for catalyzing 2-electron ORR, providing an insightful
understanding of defected carbon surface as the active catalytic sites for
2-electron ORR.