Tuning Double Layer Structure of WO3 Nanobelt for Promoting the Electrochemical Nitrogen Reduction Reaction in Water
HONG Qing-Shui, LI Tang-Yi, ZHENG Shi-Sheng, CHEN Hai-Biao, CHU Hong-Hao, XU Kuan-Da, LI Shun-Ning, MEI Zong-Wei, ZHAO Qing-He, REN Wen-Ju, ZHAO Wen-Guang and PAN Feng*
Chin. J. Struct. Chem. 2021, 40, 519-526 DOI: 10.14102/j.cnki.0254-5861.2011-2975
April 15, 2021
electrochemical nitrogen reduction reaction, zeta potential, nitrogen diffusion and transport process, WO3 nanobelts, first-principles calculations
ABSTRACT
Electrochemical
fixation of nitrogen to ammonia with highly active, highly selective and low
cost electrocatalysts is a sustainable alternative to the extremely energy- and
capital-intensive Haber-Bosch process. Herein, we demonstrate a near electroneutral
WO3 nanobelt catalyst to be a promising electrocatalyst for
selective and efficient nitrogen reduction. The concept of near electroneutral
interface is demonstrated by fabricating WO3 nanobelts with small
zeta potential value on carbon fiber paper, which ensures a loose double layer
structure of the electrode/ electrolyte interface and allows nitrogen molecules
access the active sites more easily and regulates proton transfer to increase
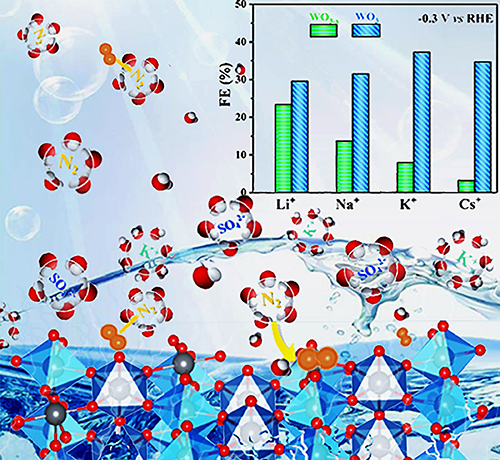
the catalytic selectivity. The WO3/CFP electrode with optimal
surface charge achieves a NH3 yield rate of 4.3 μg·h−1·mg−1 and a
faradaic efficiency of 37.3% at −0.3 V vs. RHE, rivalling the performance of the state-of-the-art nitrogen reduction
reaction electrocatalysts. The result reveals that an unobstructed
gas-diffusion pathway for continually supplying enough nitrogen to the active
catalytic sites is of great importance to the overall catalytic performance.