Hydrothermal Preparation and Photophysical Properties of a Ni(II) Complex Containing Quinoline Derivative and Phen Ligands
CHEN Hai-Hui, YI Xiu-Guang*, PAN Chang-Wang, WEN Ji-Wu and ZHANG Cong
Chin. J. Struct. Chem. 2021, 40, 501-506 DOI: 10.14102/j.cnki.0254-5861.2011-2951
April 15, 2021
hydrothermal preparation, crystal structure, photophysical properties
ABSTRACT
A
novel nickel complex [Ni2L2Phen3]·3H2O
(HL = 3-hydroxy-2-methylquioline-4-carboxylic acid) has been synthesized by a
hydrothermal approach and is structurally determined by single-crystal X-ray
diffraction. The title complex crystallizes in triclinic space group P
. Crystal
data for the title complex: C58H44N8Ni2O9, Mr = 1108.35, a = 11.8648(4), b = 12.7369(4), c =
17.0728(5) Å, α = 97.694(3), β = 96.702(2), γ = 99.566(3)°, V = 2495.66(14) Å3, Z = 2, T = 293(2) K, Dc = 1.475 g/cm3, μ(MoKα) = 0.824 mm–1, F(000) = 1140, R = 0.0757, wR = 0.2129 and GOF = 1.017. The nickel ions are
surrounded by five oxygen and six nitrogen atoms to yield two slightly
distorted octahedral geometries. Solid-state photoluminescence spectrum reveals
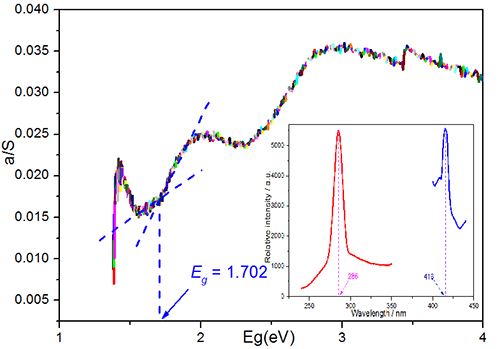
that it shows blue purple emission. Solid-state UV/Vis diffuse reflectance
spectroscopy exhibits that it has an optical band gap of 1.702 eV.