A Hexanuclear Cobalt Cluster with Tetracubane-like Topology: Synthesis, Structure and Magnetic Properties
SHI Zhen-Hai, HUANG Yuan, WU Yu-Ze, CHEN Xiao-Li and YANG Hua*
Chin. J. Struct. Chem. 2021, 40, 495-500 DOI: 10.14102/j.cnki.0254-5861.2011-2942
April 15, 2021
hexanuclear, cobalt, tetracubane, topology, magnetic property
ABSTRACT
One hexanuclear
cobalt cluster [Co2IIICo4II(L)4(CH3COO)2(MeO)4]·MeOH
(1)
was synthesized by the reaction of H2L (H2L =
2-((2-hydroxy-4-methoxy-benzylideneamino)methyl)phenol)
and Co(OAc)2·4H2O in MeOH under solvothermal conditions. Complex 1 crystalizes in the triclinic space group P with a =
14.397(3), b = 16.625(3), c = 18.992(4) Å, α = 109.47(3)°, β = 99.24(3)°, γ = 112.37(3)°, Dc = 1.464 g/cm3, Z = 2, V = 3741.7(2) Å3, the final R = 0.0781 and wR = 0.1436
for 13051
observed
reflections with I > 2σ(I). In
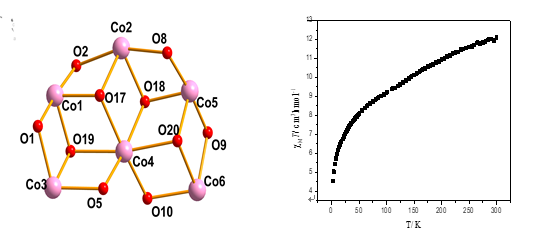
the structure of 1,
two cobalt ions are in 3+ oxidation states and four cobalt ions are in 2+
valence states. The six cobalt atoms are held together by six
phenolate oxygen atoms from four L2– ligands, four oxygen atoms from
two chelating acetates and four μ3-O
atoms from four MeO– groups. The
six cobalt atoms are located at six corners of four defective cubanes. Thus,
complex 1 displays tetracubane-like topology. Solid-state
dc magnetic susceptibilities were measured
for 1 in the 2.0~300 K range. Antiferromagnetic
interactions were determined for 1.
with a =
14.397(3), b = 16.625(3), c = 18.992(4) Å, α = 109.47(3)°, β = 99.24(3)°, γ = 112.37(3)°, Dc = 1.464 g/cm3, Z = 2, V = 3741.7(2) Å3, the final R = 0.0781 and wR = 0.1436
for 13051
observed
reflections with I > 2σ(I). In
the structure of 1,
two cobalt ions are in 3+ oxidation states and four cobalt ions are in 2+
valence states. The six cobalt atoms are held together by six
phenolate oxygen atoms from four L2– ligands, four oxygen atoms from
two chelating acetates and four μ3-O
atoms from four MeO– groups. The
six cobalt atoms are located at six corners of four defective cubanes. Thus,
complex 1 displays tetracubane-like topology. Solid-state
dc magnetic susceptibilities were measured
for 1 in the 2.0~300 K range. Antiferromagnetic
interactions were determined for 1.