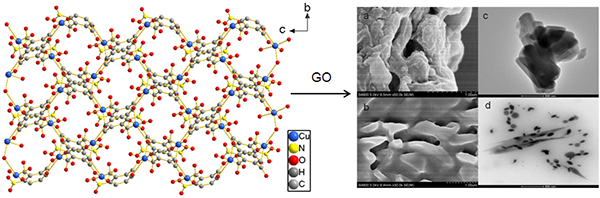
A 2D Layer Copper(II) Coordination Polymer with 3-Nitrophthalic
Acid: Synthesis, Crystal Structure and Copper 3-Nitrophthalate Metal-organic
Framework-graphene Oxide Nanocomposite
TAN Hong-Hui, LV Xiao-Long, LIU Jiang-Long, CHENG Yu-Fang, ZHOU Qing-Lin, LIN Yi-Ting and MENG Wei*
Chin. J. Struct. Chem. 2021, 40, 459-464 DOI: 10.14102/j.cnki.0254-5861.2011-2970
April 15, 2021
copper 3-nitrophthalate metal-organic framework, crystal structure, graphene oxide, nanocomposite
ABSTRACT
A 2D layer Cu(II)
coordination polymer [Cu(npth)(H2O)]n (1) was
crystallized from a mixture of 3-nitrophthalic acid
and Cu(OAc)2·H2O in water under room temperature and structurally
characterized by single-crystal X-ray diffraction, FT-IR and TGA. Compound 1 was applied to make a nanocomposite with graphene oxide (GO). A highly
dispersible and stable nanocomposite of Cu(npth)-GO was successfully synthesized by a simple ultrasonication method. SEM, TEM,
UV-vis, FT-IR and TGA were used to characterize the morphology and structure of the prepared
composite. In accordance with the characterization results, we suspected that the
binding mechanism of Cu(npth) and GO was assigned to be the cooperative
interaction of Cu–O coordination, π-π stacking and hydrogen bonding.