Hydrothermal Synthesis, Crystal Structure and Properties of a New Binuclear Nickel(III) Complex with 3-(Pyridin-2-yl)-1,2,4-triazole
LI Chang-Hong*, LI Yu-Lin*, LI Wei* and KUANG Yun-Fei
Chin. J. Struct. Chem. 2021, 40, 363-368 DOI: 10.14102/j.cnki.0254-5861.2011-2931
March 15, 2021
binuclear nickel(II) complex, thermal stability, fluorescent and magnetic properties
ABSTRACT
A new binuclear nickel(II) complex Ni2(HPT)4(H2O)3 (1) has been hydrothermally
synthesized with nickel hydroxide, 3,4-pyridine dicarboxylic acid and 3-(pyridin-2-yl)-1,2,4-triazole (HPT) in the mixed
solution (the volume ratio of methanol and water is 1:4). It crystallizes in tetragonal
space group P42/n,
with a = 20.844(1), b = 20.844(1), c = 7.2463(7) Å, V = 3148.2(5) Å3, Dc = 1.587 g/cm3, Z = 4, μ(MoKa)
= 1.257, F(000) = 1544, the final GOOF = 1.043, R = 0.0437 and wR =
0.1297. The crystal
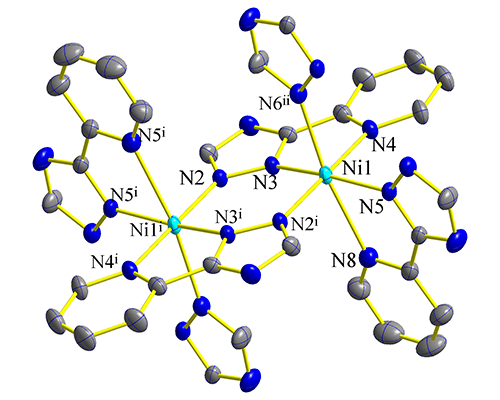
structure shows that the whole molecule consists of two nickel ions which are
bridged by four μ2-η1:η0-3-(pyridin-2-yl)-1,2,4-triazole
anions. The coordination environment of Ni(II) ion is NiN6, giving
a distorted octahedral geometry. The thermal stability and fluorescent and magnetic properties of the complex were investigated.