One Novel Ag(I) Compound Generated from Double Schiff-base Ligand with Quinoxaline-N-oxide as Terminal Binding Sites
WANG Dong-Xiao, WANG Peng* and CHENG Jun-Yan*
Chin. J. Struct. Chem. 2021, 40, 301-305 DOI: 10.14102/j.cnki.0254-5861.2011-2843
March 15, 2021
crystal structure, double Schiff-base ligand, discrete coordination compound, hydrogen bond
ABSTRACT
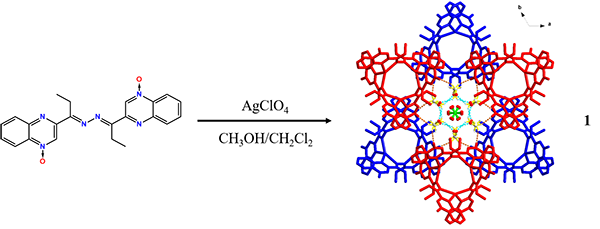
One
novel discrete coordination molecule with AgI centers, namely [Ag3(L)3(ClO4)3]∙3CH2Cl2∙6CH3OH∙1.5H2O
(1), has been synthesized based on the double
Schiff-base ligand, 3,6-bis[2-(4-oxide- quinoxaline)-yl]-4,5-diaza-3,5-octadiene
(L) and AgClO4. The obtained Ag(I) compound was fully characterized
by infrared spectroscopy, elemental analysis, and single-crystal X-ray
diffraction. 1 is of trigonal system space group P-31c with a = 16.320(2), b = 16.320(2), c = 20.108(6) Å, T = 173(2) K, V = 4638.2(16) Å3, Dc = 1.645 g/cm3, Mr = 2297.33, Z = 2, F(000) = 2334, μ = 0.970 mm–1, Goodness-of-fit = 1.109, the final R = 0.0776, wR = 0.1813, R indices (all data) = 0.1196, wR = 0.2011. The compound exhibits a triple-helical [Ag3L3]3+ crown-like
trimer, in which three Ag(I) atoms form an equilateral triangle with the Ag···Ag
distance of 4.7 Å. Uncoordinated counterions ClO4– and solvent molecules methanol
generate the hydrogen-bonded frameworks based on discrete molecular complex
building blocks.