Synthesis, Structure and Characterization of a New Silicophosphate, K2SiP4O13, with a Six-fold Coordinated Si
SUN Tong-Qing*, WANG Qian-Qian, KONG Yong-Fa and XU Jing-Jun
Chin. J. Struct. Chem. 2021, 40, 256-263 DOI: 10.14102/j.cnki.0254-5861.2011-2851
February 15, 2021
synthesis, silicophosphate, SiO6 octahedral coordination, crystal structure
ABSTRACT
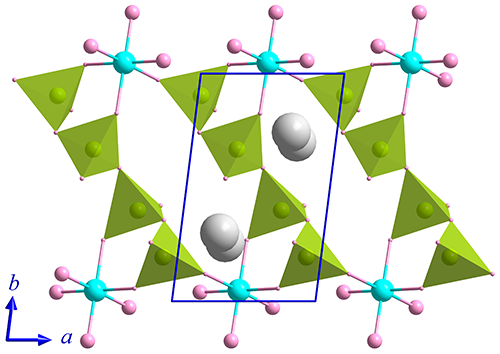
A potassium silicophosphate,
K2SiP4O13, has been synthesized in molten
polyphosphoric acid. It crystalizes in the triclinic space group P
(No. 2) with a = 4.8327(10), b = 7.7403(15), c =
14.485(3) Å, a = 82.29(3)°, b = 83.31(3)°, g = 81.95°, V = 529.02(19) Å3, Z = 2. The crystallographic structure features
2D layers of [SiP4O13]¥ in the ab plane with counter cations K+ residing among the layers, and the anionic framework of [SiP4O13]¥ is composed of six-fold coordinated Si atoms and tetraphosphate anions by
sharing vertex O atoms. The title compound was characterized by powder X-ray
diffraction, IR and Raman spectroscopies, UV-vis diffuse
reflectance spectroscopy, thermogravimetry and
differential scanning calorimetry.