Synthesis, Crystal Structures and Cytotoxic Activities of Two New Pyrimidine Derivatives of Ursolic Acid
WANG Wen-Yan, LI A-Liang, LIU Qing-Song, SUN Yue and GU Wen*
Chin. J. Struct. Chem. 2021, 40, 239-245 DOI: 10.14102/j.cnki.0254-5861.2011-2818
February 15, 2021
ursolic acid, pyrimidine, crystal structure, cytotoxic activity
ABSTRACT
The title compounds (5a and 5b) were
synthesized from ursolic acid and their structures were characterized by
spectroscopic methods including ESI-MS, 1H-NMR, 13C-NMR
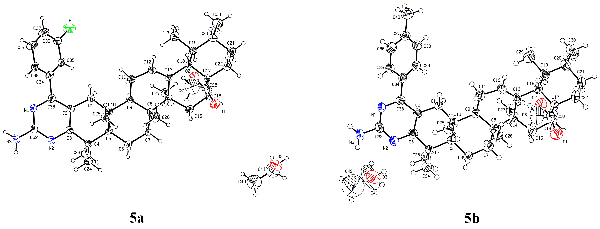
and elemental analysis. The crystal structures of compounds 5a and 5b were determined by single-crystal X-ray diffraction analysis.
Compound 5a crystallizes in
monoclinic system, P21 space group with a = 12.258(3), b = 10.396(2), c = 15.570(3) Å, b = 107.21(3)°, Z = 2, V = 1895.3(7) Å3, Mr = 659.90, Dc = 1.156 Mg/m3, S = 1.003, µ = 0.076 mm−1, F(000) = 716, the final R = 0.0686 and wR = 0.1430 for 1859 observed reflections (I > 2σ(I)). Compound 5b crystallizes in monoclinic system, P21 space group with a = 12.371(3), b = 10.647(2), c = 15.722(3) Å, b = 109.44(3)°, Z = 2, V = 1952.8(8) Å3, Mr = 655.93, Dc = 1.116 Mg/m3, S = 1.002, µ = 0.069 mm−1, F(000) = 716, the final R = 0.0686 and wR = 0.1882 for 2574 observed reflections (I > 2σ(I)). The preliminary cytotoxic assay
indicated that compound 5b exhibited
notable cytotoxic activity against MCF-7 and HeLa cells with the IC50 values of 10.71 ± 0.23 and 12.63 ± 0.31 μM, respectively.