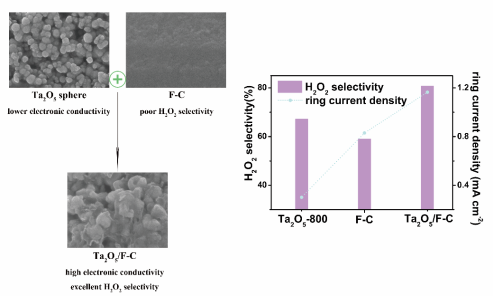
Synergistic Effect of Ta2O5/F-C Composites for Effective Electrosynthesis of Hydrogen Peroxide from O2 Reduction
WANG Ke, PANG Yong-Yu, XIE Huan, SUN Yuan and CHAI Guo-Liang*
Chin. J. Struct. Chem. 2021, 40, 225-232 DOI: 10.14102/j.cnki.0254-5861.2011-2817
February 15, 2021
Ta2O5/F-C, oxygen reduction reaction, hydrogen peroxide
ABSTRACT
The electrosynthesis of H2O2 as
an environmentally friendly green process has attracted great attention due to
the importance of H2O2 in industry and human lives. In
this work, a new strategy was proposed to improve the electrical conductivity
and H2O2 selectivity of transition metal oxides
catalysts. F-C (F doped carbon) was coupled with Ta2O5 by
calcining polyvinylidene fluoride (PVDF) as the carbon source using one step
method. The Ta2O5/F-C composite catalysts
show an excellent H2O2 selectivity of more than 80% as
well as high reactivity at 2.52 mA/cm2, which is greatly enhanced
compared to the counterparts of F-C (selectivity of 59%) and Ta2O5-800
(current density of 0.85 mA/cm2) in 0.1 M KOH solution. The onset
potential for H2O2 production on Ta2O5/F-C composites is 0.78 V in 0.1 M KOH, which indicates a negligible
overpotential. In addition, H2O2 selectivity of the
catalyst can be stabilized at more than 80% after 10 hours of electrolysis in
alkaline electrolyte. The high performance due to the introduction of F-C increases the conductivity of Ta2O5 and the
synergistic effect between F-C and Ta2O5. This work proposed
an efficient synergistic effect among F-doped C and Ta2O5 for H2O2 production.