Enhanced CO2 Electrolysis with Metal-Oxide Interface Structures
XU Ze-Tong and XIE Kui*
Chin. J. Struct. Chem. 2021, 40, 31-41 DOI: 10.14102/j.cnki.0254-5861.2011-2744
January 15, 2021
solid oxide electrolyser cell, LSCM, metal nanoparticles, CO2 electrolysis
ABSTRACT
The
ever-decreasing fossil fuels and the increasing greenhouse effect have caused substantial
concern. Solid oxide electrolyser cell (SOEC) with La0.75Sr0.25Cr0.5Mn0.5O3-δ (LSCM) as a cathode was used for CO2 electrolysis to CO. In this work,
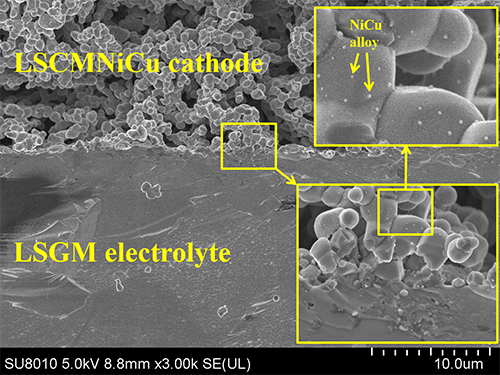
the metal-oxide interface was constructed on the LSCM framework by in-situ exsolution and impregnation, and
the uniform distribution of metal nanoparticles on the LSCM framework was confirmed
by spectroscopy techniques and electron microscopy techniques. The existence of
three-phase boundary promoted
the absorption and electrolysis of CO2. (La0.75Sr0.25)0.9(Cr0.5Mn0.5)0.9(Ni0.5Cu0.5)0.1O3-δ (LSCMNC) showed the best electrolytic CO2 performance at 850 ℃ and exhibited excellent electrocatalytic activity after 100 hours of
long-term testing and 8 redox cycles.