Cover Picture
Ultrathin ZnIn2S4 Nanosheets
Supported Metallic Ni3FeN for Photocatalytic Coupled Selective
Alcohol Oxidation and H2 Evolution
Mengqing Li, Weiliang Qi, Jiuyang Yu, Lijuan Shen, Xuhui Yang, Siqi Liu* and Min-Quan Yang*
Submit a ManuscriptThe Structural and Chemical Reactivity of Lattice Oxygens on β-PbO2 EOP Electrocatalysts
Wenwen Li, Ge Feng, Jia Liu, Xing Zhong, Zihao Yao, Shengwei Deng, Shibin Wang* and Jianguo Wang
Chin. J. Struct. Chem. 2022, 41, 2212051-2212059 DOI: 10.14102/j.cnki.0254-5861.2022-0153
December 2, 2022
oxygen vacancy effect, electrochemical ozone production, lattice oxygen mechanism, density functional theory
ABSTRACT
The oxygen evolution reaction (OER) and electrochemical
ozone production (EOP) attracted considerable attention due to their
wide applications in electrocatalysis, but the detailed reaction mechanism of
product formation as well as the voltage effect on O2/O3 formation still remains unclear. In this work, the density functional theory
calculations were used to systematically investigate the possible reaction
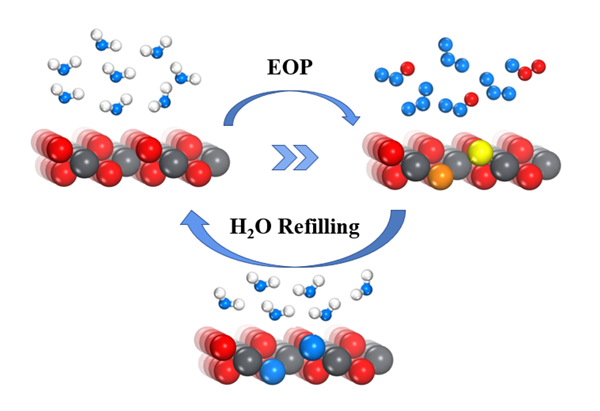
mechanisms of OER and EOP on the PbO2 (110) surface, with the
possible reaction network involving surface lattice oxygen atoms (LOM)
proposed. The results show that the LOM-2 reaction pathway involving two
surface lattice oxygen atoms (Olatt) and one oxygen atom from H2O
was the most thermodynamically reactive. Different potential determining step
(PDS) was obtained depending on the multiple reaction pathway, and the results
show that the facile diffusion of Olatt would proceed the LOM
pathway and promote the formation of surface oxygen vacancies (Ovac1/Ovac2). Furthermore, Ovac1/Ovac2 formation on the surface would trigger further reactions of H2O
adsorption and splitting, which refilled the oxygen vacancy and ensured the
considerable stability of the PbO2 (110) surface. Multiple H2O
dissociation pathways were proposed on PbO2 (110) with oxygen
vacancy sites: the acid-base interaction mechanism and the vacancy fulfilling
mechanism.