Control of pore environment in nickel-based metal-organic frameworks for SF6/N2 separation
Hao-Ran Liu, Shao-Min Wang, Yong-Li Dong, Su-Tao Zheng, Shuang Ni, Jie Xu, Qing-Yuan Yang*
Chin. J. Struct. Chem., 2023, 42: 100022. DOI: 10.1016/j.cjsc.2023.100022
February 15, 2023SF6/N2 separation, Greenhouse gas, Metal-organic frameworks, Adsorption
ABSTRACT
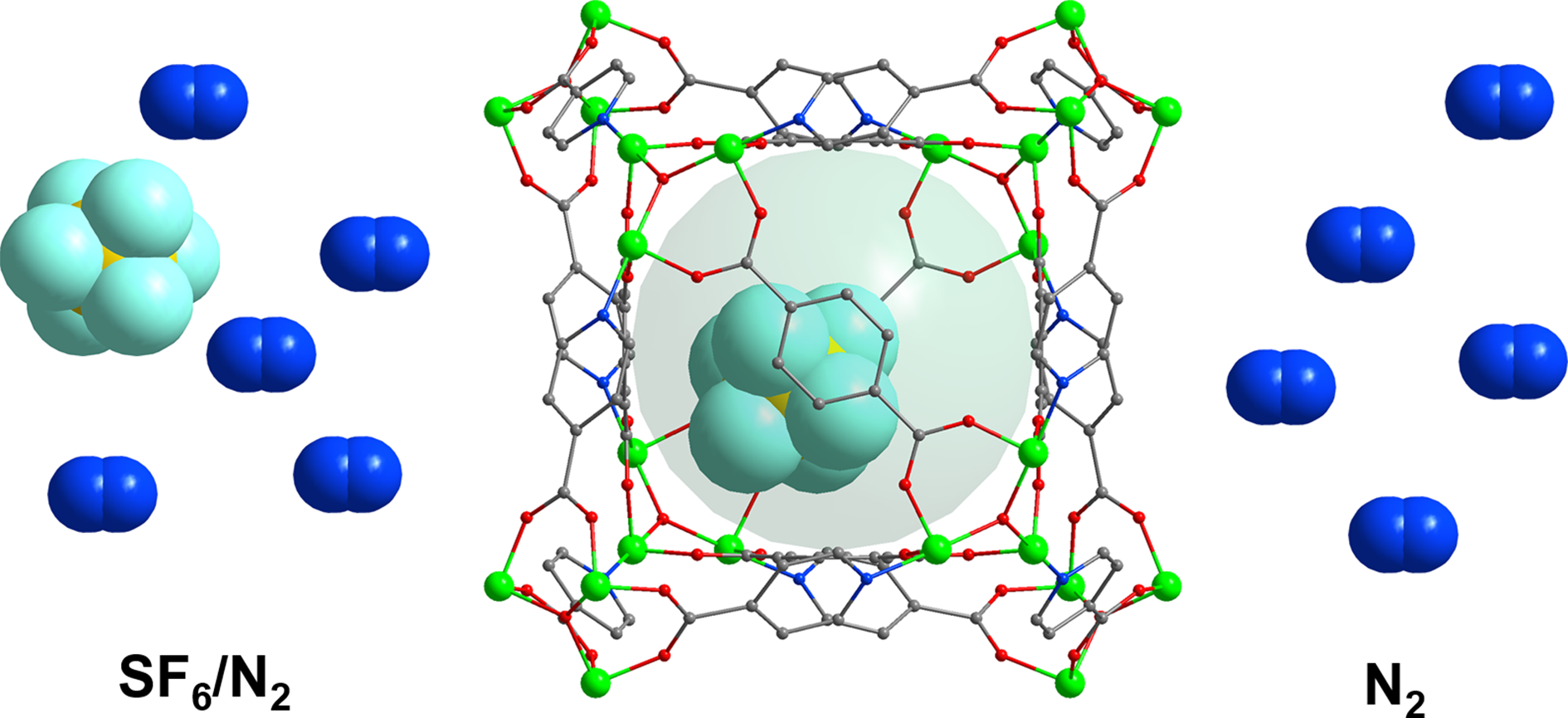
In the semiconductor industry, recovering and capturing SF6 gas from the SF6/N2 gas mixture has significant environmental and economic benefits. In this work, two Ni-based metal-organic frameworks (MOFs), Ni(ina)(bdc)0.5 (ina = isonicotinic acid, bdc = terephthalic acid) and methyl-functionalized Ni(3-min)(bdc)0.5 (3-min = 3-methylisonicotinic acid), are constructed for SF6 capture. Ni(ina)(bdc)0.5 and Ni(3-min)(bdc)0.5 are both highly stable nine-connected porous materials. The pore sizes of these two materials are similar, but the pore environments are different, which has a bearing on the performance of SF6/N2 separations. Ni(3-min)(bdc)0.5 exhibits higher SF6 adsorption capacity (50.5 cm−3 g−1) and IAST (ideal adsorbed solution theory) SF6/N2 selectivity (91) owing to its smaller window diameter and suitable pore chemistry. Theoretical calculations indicate that the SF6 and N2 molecules interact with the framework at different cages, which reduces their competition for adsorption sites. The remarkable separation performance of Ni(ina)(bdc)0.5 and Ni(3-min)(bdc)0.5 is further verified by dynamic breakthrough experiments. Thus, these two adsorbents have the potential to be utilized in industrial applications due to their excellent structural stability and recyclability.