Cover Picture
Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries
Renshu Huang, Jinli Chen, Xingfa Chen, Tianqi Yu, Huyi Yu, Kaien Li, Bin Li*, Shibin Yin* Submit a Manuscript
Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries
Renshu Huang, Jinli Chen, Xingfa Chen, Tianqi Yu, Huyi Yu, Kaien Li, Bin Li*, Shibin Yin* Submit a Manuscript
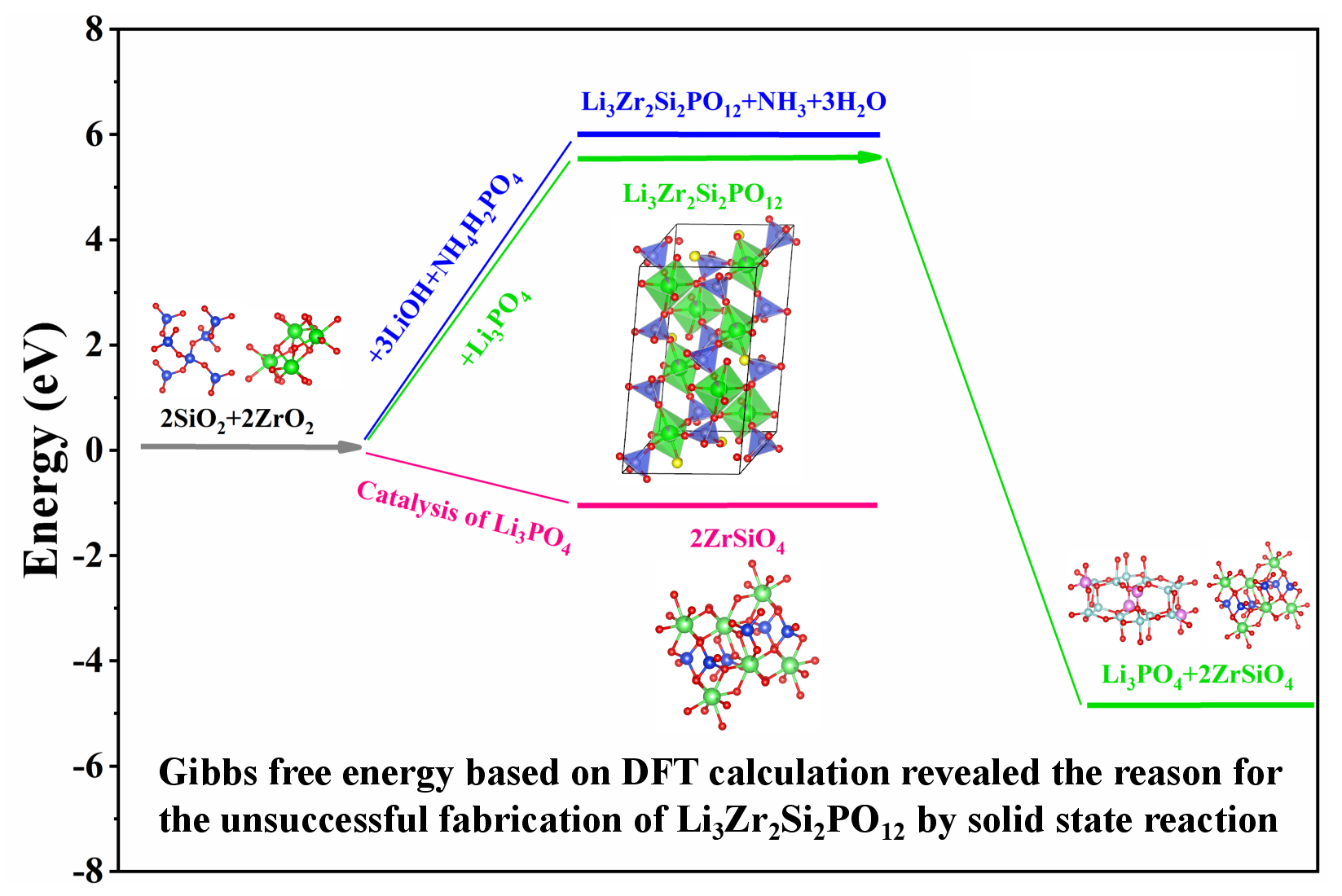
Revealing the reason for the unsuccessful fabrication of Li3Zr2Si2PO12 by solid state reaction
Zizhuo Liang, Fuming Du, Ning Zhao*, Xiangxin Guo*
Chin. J. Struct. Chem., 2023, 42: 100108. DOI: 10.1016/j.cjsc.2023.100108
November 15, 2023
NASICON-type solid electrolytes; Na3Zr2Si2PO12; Li3Zr2Si2PO12; Thermodynamic analysis; Coordination structure
ABSTRACT
NASICON type Li3Zr2Si2PO12 can be synthesized via cation exchange method with Na3Zr2Si2PO12 as precursor, which retains the skeleton structure and achieves an ionic conductivity higher than 3 mS cm−1 at room temperature. However, large-scale fabrication via cation exchange reaction seems unlikely considering the expensive precursors and complicated preparation process. Herein, the viability of solid-state reaction to prepare Li3Zr2Si2PO12 is explored, which has important implication for its industrialization. The sintering was conducted using the raw materials of LiOH, SiO2, ZrO2 and NH4H2PO4 with the nominal stoichiometric ratio of Li3Zr2Si2PO12. The results show that the final product is a Li3PO4·2ZrSiO4 composite with negligible Li+ conductivity, other than the expected Li3Zr2Si2PO12 with high Li+ conductivity. Combined with thermodynamic calculations based on density functional theory (DFT), the competition between Li3PO4·2ZrSiO4 and Li3Zr2Si2PO12 with NASICON phase is analyzed. It was found that the formation energy (ΔG) of Li3PO4·2ZrSiO4 is lower than that of Li3Zr2Si2PO12. In addition, the decomposition of Li3Zr2Si2PO12 with Li3PO4·2ZrSiO4 as products is a thermodynamically spontaneous reaction. The influences related to the coordination structures on the structural stability of NZSP are discussed as well. These results demonstrate that the fabrication of Li3Zr2Si2PO12 through high-temperature sintering is difficult, and the development of a synthetic method with mild conditions is essential for the Li3Zr2Si2PO12 preparation.