Cover Picture
Metal-ion-tuned metal-organic frameworks for C2H2/CO2 separation
Meng Sun, Hongyan Liu, Xiaokang Wang, Xinlei Yang, Fei Gao, Deyu Xie, Weidong Fan*, Yinfeng Han*, Ben Xu, Daofeng Sun Submit a Manuscript
Metal-ion-tuned metal-organic frameworks for C2H2/CO2 separation
Meng Sun, Hongyan Liu, Xiaokang Wang, Xinlei Yang, Fei Gao, Deyu Xie, Weidong Fan*, Yinfeng Han*, Ben Xu, Daofeng Sun Submit a Manuscript
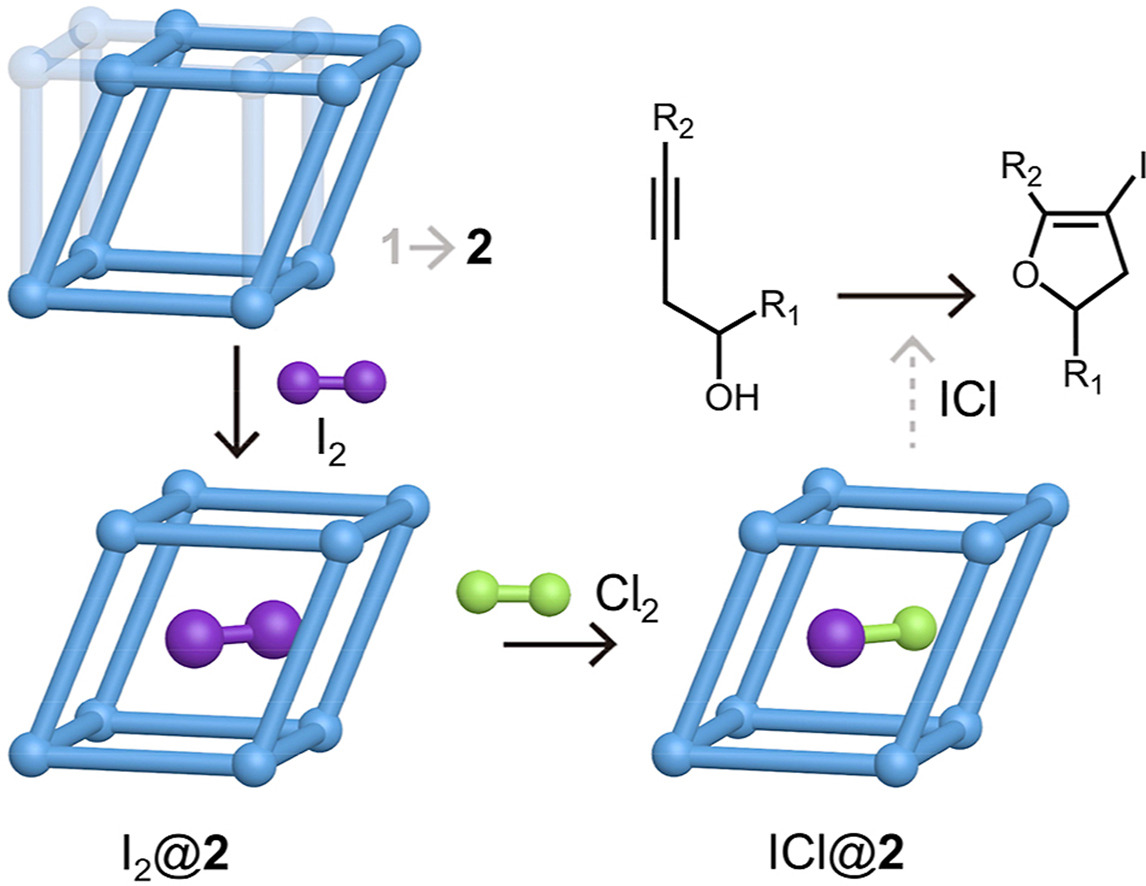
A Cd-based MOF crystal vessel for the synthesis of interhalogens
Min-Jie Zhang, Yu Ge, Chen Cao, Xin-Ran Xue, Qiu-Yi Li, Qi Liu*, Jian-Ping Lang*
Chin. J. Struct. Chem., 2023, 42: 100125. DOI: 10.1016/j.cjsc.2023.100125
September 15, 2023
Metal-organic frameworks; Crystal vessel; Interhalogens; Crystal structures; Iodocyclization
ABSTRACT
As important halogenating and oxidant agents, the synthesis and utilization of interhalogens are plagued by their purity problem, strong volatility and high reactivity. Herein, a flexible Cd-based metal-organic framework (MOF), {[Cd(1,4-bdc)(4-bpa)]·DMF}n (1, where 4-bpa = 1,2-bis(4-pyridyl)acetylene), was prepared and its corresponding activation species, [Cd(1,4-bdc)(4-bpa)]n (2), with moderate pore size and shape, acting as a crystal vessel, was applied to synthesize and store pure interhalogens. The synthesis of interhalogen was realized by quantitive transformation of halogen molecules incorporated in the pores of 2, which was confirmed by single-crystal X-ray diffraction and other structural characterizations. The embedded interhalogen molecules were stabilized by their interactions with the inner groups of the porous framework of 2 and released in polar solvent and utilized in iodocyclization of organic alcohols with high selectivity. This work not only opens a new door to the synthesis of pure interhalogens but also demonstrates powerful applications of MOF crystal vessels in realizing classic but important inorganic and organic reactions.