Cover Picture
Metal-ion-tuned metal-organic frameworks for C2H2/CO2 separation
Meng Sun, Hongyan Liu, Xiaokang Wang, Xinlei Yang, Fei Gao, Deyu Xie, Weidong Fan*, Yinfeng Han*, Ben Xu, Daofeng Sun Submit a Manuscript
Metal-ion-tuned metal-organic frameworks for C2H2/CO2 separation
Meng Sun, Hongyan Liu, Xiaokang Wang, Xinlei Yang, Fei Gao, Deyu Xie, Weidong Fan*, Yinfeng Han*, Ben Xu, Daofeng Sun Submit a Manuscript
Enhanced ethane/ethylene separation based on metal regulation in zeolitic imidazolate frameworks
Qi Wang, Dandong Ning, Hongwei Chen, Yang Chen*, Jinping Li, Libo Li*
Chin. J. Struct. Chem., 2023, 42: 100147. DOI: 10.1016/j.cjsc.2023.100147
September 15, 2023
ZIFs; Ethane/ethylene separation; Metal modulation; Stable structure; Pore size
ABSTRACT
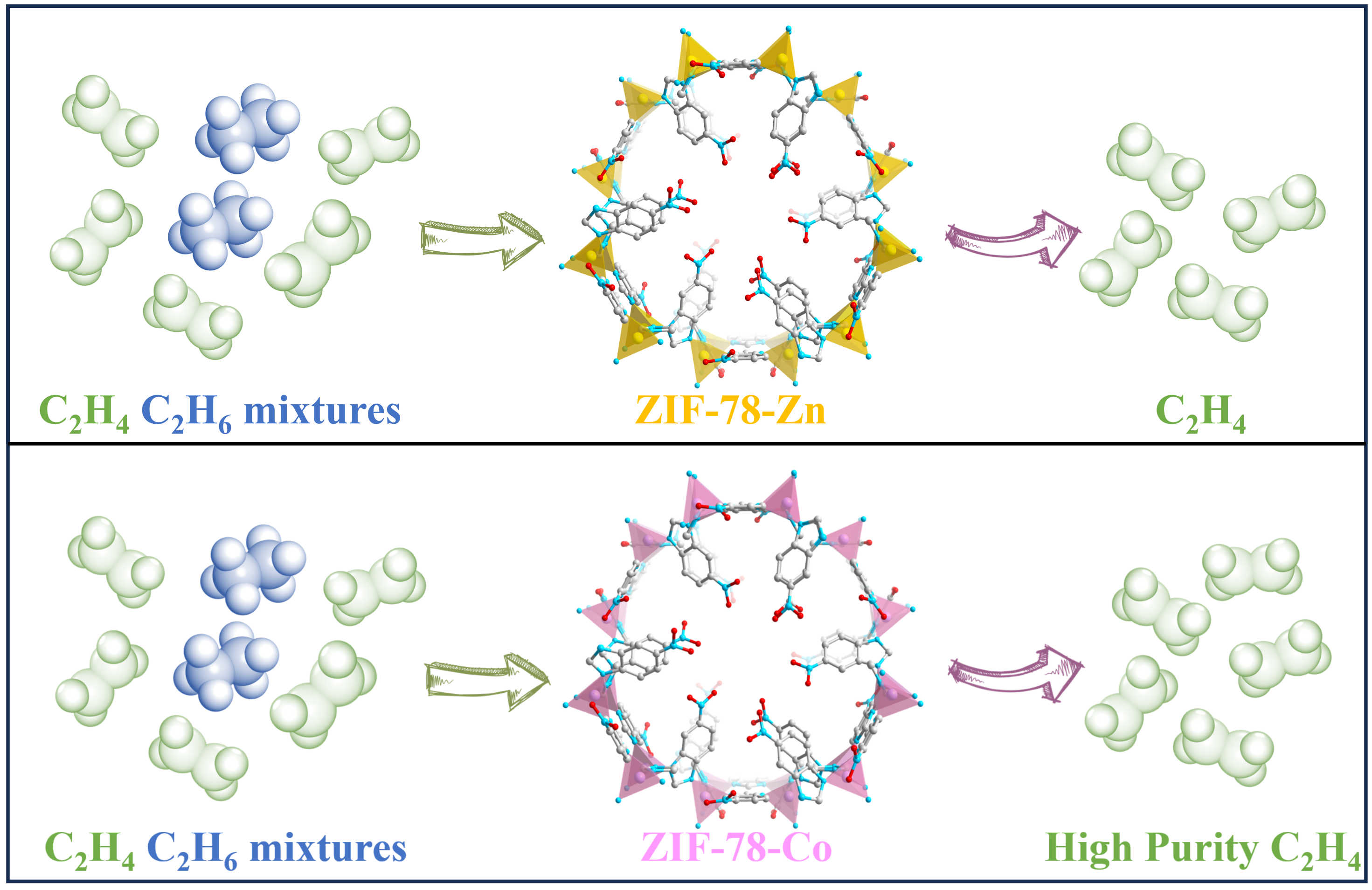
The acquisition of polymer-grade (≥ 99.95%) C2H4 poses a challenge due to the presence of ethane (C2H6) having similar physical and chemical properties. Consequently, the one-step purification of C2H4 becomes a crucial and demanding process. In this study, we synthesized ZIF-78 with a GME configuration using different metal sources (Zn, Co). Both substances have been identified as ethane-selective adsorbents with excellent thermal stability. The Brunauer Emmett Teller (BET) surface area of ZIF-78-Co (748 m2/g) surpasses that of ZIF-78-Zn (585 m2/g), and the former exhibits a higher Qst value for C2H6, resulting in enhanced adsorption capacity for C2H6 (50.61 cm3/g) and selectivity for C2H6/C2H4 (1.71) compared to ZIF-78-Zn (48.97 cm3/g, 1.46) at 298 K and 1 bar. Grand Canonical Monte Carlo (GCMC) calculations indicate that C2H6 has a stronger interaction with the ZIF-78-Co framework. Breakthrough experiments for the C2H6/C2H4 (50:50, V/V) mixture at 298 K and 1 bar demonstrate that ZIF-78-Co achieves separation in approximately 5 min/g, outperforming ZIF-78-Zn. And the separation time for ZIF-78-Co in the C2H6/C2H4 (10:90, V/V) mixture is 9 min/g. Furthermore, ZIF-78-Co exhibits excellent structural stability, thermal stability, water stability, and acid-base stability. Therefore, it holds promising prospects for practical industrial separation. Additionally, we hope that our findings inspire further experimentation on alternative metal ethane adsorbents.