Cover Picture
Submit a Manuscript
Magnetic
field-assisted microbial corrosion construction ironsulfides incorporated
nickel-iron hydroxide towards efficientoxygen evolution
Xianzheng Zhang, Yana Chen, Zhiyong Ye, Huilin Hu, Ling Lei, Feng You, Junlong Yao, Huan Yang*, Xueliang Jiang*
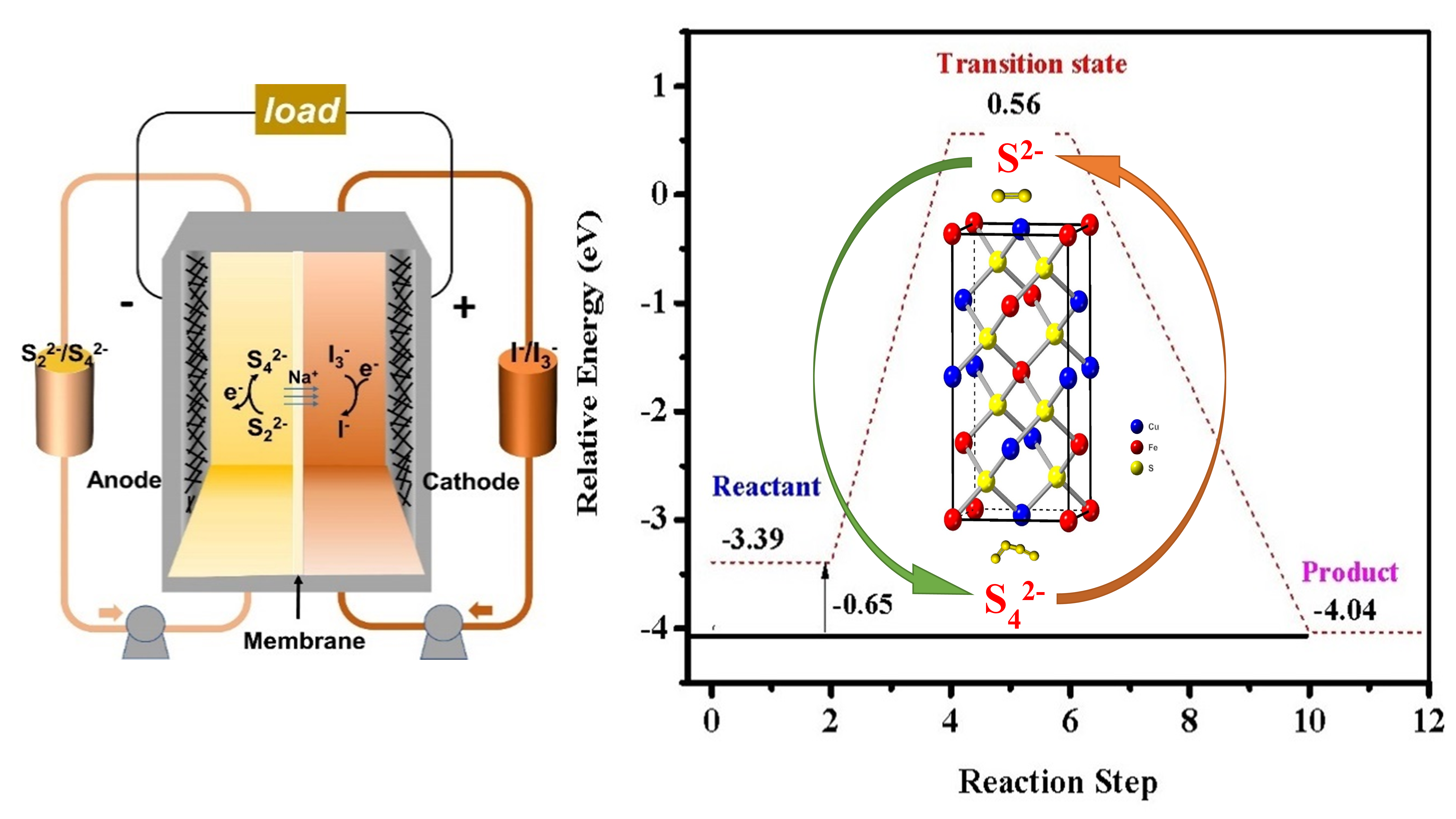
Earth-abundant CuFeS2 nanocrystals@graphite felt electrode for high performance aqueous polysulfide/iodide redox flow batteries
Tsegaye Tadesse Tsega, Jiantao Zai, Chin Wei Lai*, Xin-Hao Li*, Xuefeng Qian*
Chin. J. Struct. Chem., 2024, 43: 100192. DOI: 10.1016/j.cjsc.2023.100192
January 15, 2024
CuFeS2; Hot injection; Flow battery; Nanomaterial; DFT
ABSTRACT
Due to their high energy density and low price, aqueous polysulfide/iodide redox flow batteries are appealing for scalable energy storage. However, the greatest barrier to their practical uses is the low electrochemical kinetics of the redox reactions of polysulfide ions on graphite electrodes, which often limit their energy efficiency and power density. In this study, the CuFeS2 nanomaterial was successfully synthesized through the hot injection method, and CuFeS2 nanomaterials were uniformly coated onto the surfaces and sandwiched into graphite felt (GF); this process can significantly boost the electrocatalytic activities of S2−/Sx2− redox reactions by improving the charge transfer process, which has been proven by the electrochemical measurement and density functional theory (DFT) simulations. The polysulfide-iodide flow battery, with GF-CuFeS2 serving as the negative electrode, can achieve a high energy efficiency of 79.6% at 20 mA/cm2, a power density of 50.7 mW/cm2, and a stable energy efficiency retention of 87.0% after 180 cycles.