List of Issues>2024>February 2024
| Vol. 43, Issue 2
>
Short Communication
< PREV
Short Communication
NEXT >
Enhancing CO2 cycloaddition through ligand functionalization: A case study of UiO-66 metal-organic frameworks
Ruowen Liang*, Chao Zhang, Guiyang Yan*
Chin. J. Struct. Chem., 2024, 43: 100211. DOI: 10.1016/j.cjsc.2023.100211
February 15, 2024
ABSTRACT
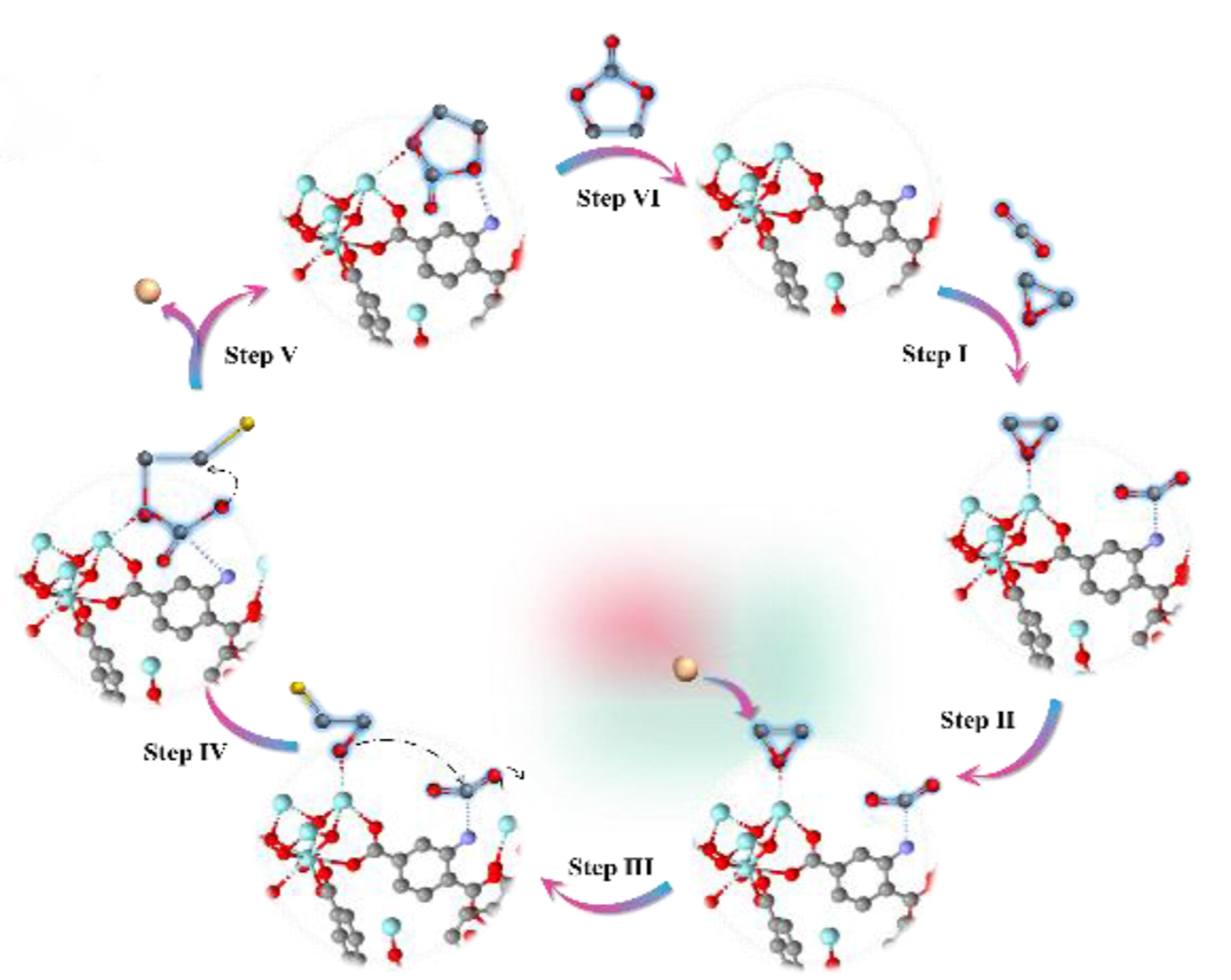
In conclusion, employing the isoreticular series of UZr-X as model systems, the UZr–NH2 exhibits the highest activity, achieving a yield of 81% after 10 h of reaction. This performance surpasses that of aminomodified MTi-NH2 and MIn-NH2, which can be attributed to the optimal electronegativity of Zr4+. DRFTIR analyses confirmed the generation of activated CO2– species during the reaction. Concurrently, the formation of NH2–CO2 and N–CO2 coordination models has been validated through theoretical calculations. Thus, in conclusion, the amino substituent and exposed zirconium sites are the crucial active sites for this reaction. In essence, the interplay between the Lewis acid/base active site and the adsorption-coordination effect culminates in the superior performance observed in CO2 cycloaddition.