Yi Zhang*, Biao Wang, Chao Hu, Muhammad Humayun, Yaping Huang, Yulin Cao, Mosaad Negem, Yigang Ding, Chundong Wang*
Chin. J. Struct. Chem., 2024, 43: 100243. DOI: 10.1016/j.cjsc.2024.100243
February 15, 2024
Fluoride; Oxygen evolution reaction; Fe–Ni–F; Reaction kinetics
ABSTRACT
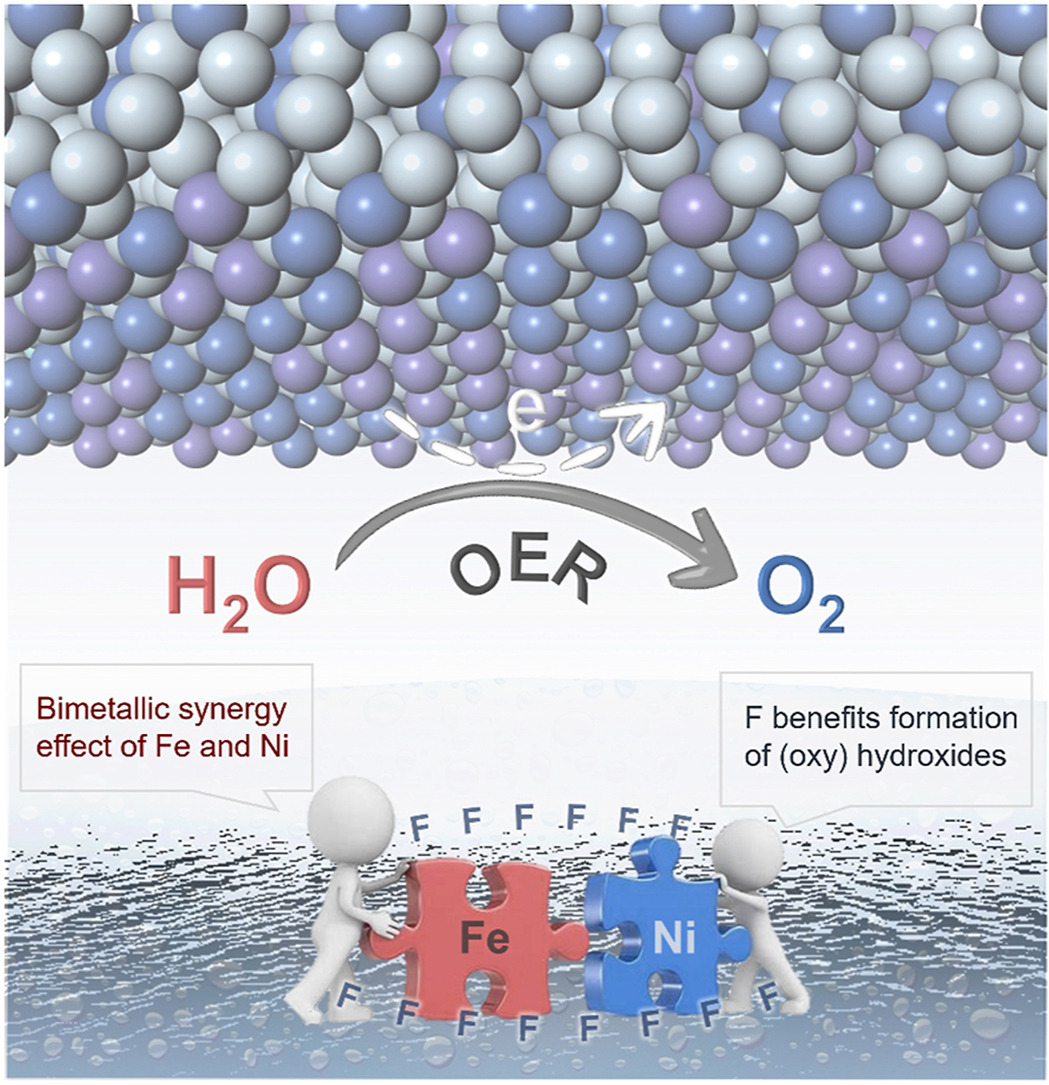
Highly active and low-cost oxygen evolution reaction (OER) catalytic electrodes are extremely essential for exploration of green hydrogen via water splitting. Herein, an advanced Fe–Ni–F electrocatalyst is fabricated by a facile annealing strategy using ammonium fluoride, of which the structure feature is unveiled by XRD, FESEM, TEM, EDS, BET, and XPS measurements. The as-prepared Fe–Ni–F addresses a low overpotential of 277 mV and a small Tafel slope of 49 mV dec−1 at a current density of 10 mA cm−2, significantly outperforming other control samples as well as the state-of-the-art RuO2. The advanced nature of our Fe–Ni–F catalyst could also be further evidenced from the robust stability in KOH alkaline solution, showing as 5.41% degradation after 24 h continuous working. Upon analysis, it suggests that the decent catalytic activity should be attributed to the formed bimetallic (oxy)hydroxides because of the introduction of fluoride and the synergistic effect of iron and nickel towards oxygen generation. This work represents the potential of Fe- and/or Ni-based fluoride as efficient catalyst for low-energy consumption oxygen generation.