Cover Picture
Progress and
Understanding on Catalysts with Well-Defined Interface for Boosting CO2 Conversion
Binran Zhao, Yiyi Zhao, Peng Liu, Yulong Men, Xinyu Meng and Yunxiang Pan*
In the cover picture, we adapt
the ideal from "Journey to the West," one of Chinese four literary
classics. The ancient Chinese mythical character, Monkey King, holds a treasure
in his hand, which represents the catalysts with well-defined interface. CO2 is blown out from the mouth of Monkey King, then goes through the catalyst
interface, and finally is converted into high value-added chemicals like CH4,
CH3OH and CO. This well describes the focus of our paper titled “Progress
and Understanding on Catalysts with Well-defined Interface for Boosting CO2 Conversion”.
Submit a Manuscript
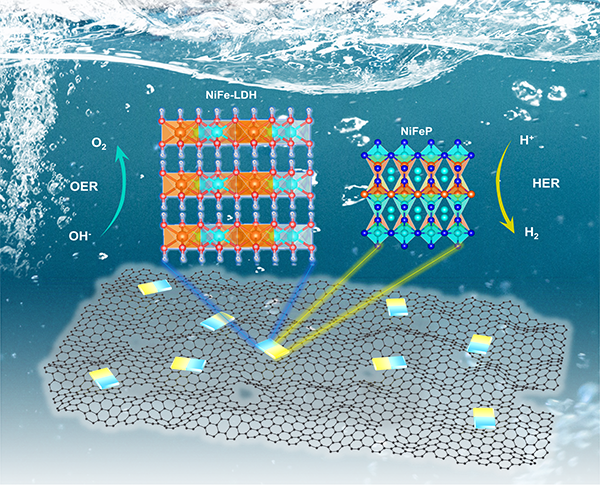
Interfacial Engineering of NiFeP/NiFe-LDH Heterojunction for Efficient Overall Water Splitting
Xuanyu Long, Jiazhi Meng, Jiabao Gu, Lanqing Ling, Qianwen Li, Nan Liu, Kaiwen Wang* and Zequan Li*
Chin. J. Struct. Chem. 2022, 41, 2204046-2204053 DOI: 10.14102/j.cnki.0254-5861.2022-0048
April 8, 2022
overall water splitting, electrocatalysis, heterojunction, phosphide, layered double hydroxide
ABSTRACT
In consideration of application prospect of non-noble metallic materials
catalysts, the study of exploring more highly effective electrocatalysts has
been focused on by researchers. Herein, a novel strategy is employed to
construct a heterojunction consisting of metal phosphide NixFeyP
and layered double hydroxide (LDH) with graphene oxide (GO) as conductive
support. By adjusting the molar ratio of Ni to Fe, a series of heterojunctions
with mixed valence state Feδ+/Fe3+ and Niδ+/Ni2+ (δ is likely close to 0) redox couples are
achieved and strong synergistic effects towards overall water splitting
performance are found. The optimized catalyst with a Ni/Fe molar ratio of
0.72:0.33, namely Ni0.7Fe0.3P/LDH/GO, delivers ultra-low
overpotentials for hydrogen evolution reaction (HER) and oxygen evolution
reaction (OER) of 79 and 198 mV at the current density of 10 mA·cm-2,
respectively. Furthermore, for overall water-splitting practical application,
it only requires 1.526 V at 10 mA·cm-2 with robust stability, which
is superior to most reported electrocatalysts. Experimental results demonstrate
the improved electronic conductivity, enlarged electrochemically active area
and accelerated kinetics together account for the enhanced performance. This
work supplies new prospects for the promotion and application of such
heterojunction electrocatalysts in overall water splitting.