Cover Picture
Progress and
Understanding on Catalysts with Well-Defined Interface for Boosting CO2 Conversion
Binran Zhao, Yiyi Zhao, Peng Liu, Yulong Men, Xinyu Meng and Yunxiang Pan*
-
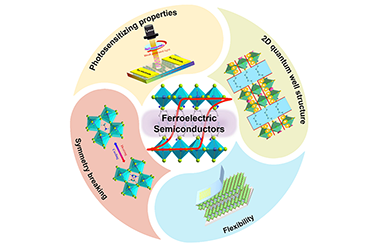
ReviewResearch Advances of Ferroelectric Semiconductors of 2D Hybrid Perovskites toward Photoelectronic Applications
Yaoyao Chen, Changhao Gao, Tian Yang, Wenjing Li, Haojie Xu and Zhihua Sun*
Chin. J. Struct. Chem. 2022, 41, 2204001-2204011. DOI: 10.14102/j.cnki.0254-5861.2022-0013
April 8, 2022
ferroelectric materials, hybrid perovskites, semiconductor properties, two-dimensional
ABSTRACT
Ferroelectric materials, characterized by the switchable spontaneous polarization (Ps) through reversing the directions of external electric field, exhibit versatile physical attributes that have been extensively used for practical device applications. Two-dimensional (2D) organic-inorganic hybrid perovskites are recently emerging as a robust family of candidate ferroelectrics, termed ferroelectric semiconductors. In particular, the coexistence and/or coupling of ferroelectric polarization with their semiconducting properties enables new physical concepts, thus providing a potential platform for the development of new multifunctional optoelectronic devices. This review primarily describes the structural origin of symmetry breaking for generating ferroelectric orders in 2D hybrid perovskites, and then presents the combination of ferroelectric Ps with other semiconducting optoelectronic activities. Regarding the emergence of new photoelectric behaviors, the prospects for this 2D family of ferroelectric semiconductors are further discussed, along with their development tendency for the future photoelectronic device applications. -
Review
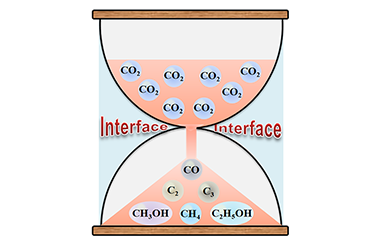
Progress and Understanding on Catalysts with Well-Defined Interface for Boosting CO2 Conversion
Binran Zhao, Yiyi Zhao, Peng Liu, Yulong Men, Xinyu Meng and Yunxiang Pan*
Chin. J. Struct. Chem. 2022, 41, 2204012-2204021 DOI: 10.14102/j.cnki.0254-5861.2022-0024
April 8, 2022
carbon dioxide, heterogeneous catalysis, interface effect, noble metal, transition metal, cold plasma
ABSTRACT
Catalytic conversion of CO2 into valuable chemicals like CH3OH is the most promising way to alleviate CO2 emission for solving the serious climate change issue. Multi-component catalysts with well-defined interface show outstanding performance in CO2 conversion due to the synergistic effects and multifunctional properties caused by the well-defined interface. A discharge technique, named as cold plasma, has been recognized as an excellent strategy for tuning catalyst interface properties. The temperature of cold plasma is lower than 200 ⁰C, and can be further lowered to room temperature by simply changing the operation conditions of cold plasma. The lower temperature of cold plasma can well maintain the catalyst structures, especially the porous structures. When conducting cold plasma, in addition to nontoxic working gases like Ar and air, no harmful substances are used. Cold-plasma-prepared catalysts have unique interface properties, and thereby exhibit superior performance in CO2 conversion over the catalysts prepared by traditional methods. The present review summarizes the progress about the cold-plasma-prepared catalysts for CO2 conversion, discusses the origin for the outstanding catalytic performance, and proposes the challenges and opportunities for further studies. This will stimulate more deep insights into the cold-plasma-prepared catalysts with well-defined interface properties for achieving more efficient CO2 conversion. -
Review
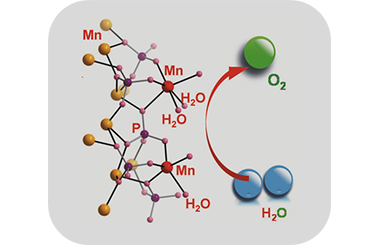
The Mechanism of Water Oxidation from Mn-Based Heterogeneous Electrocatalysts
Shujiao Yang, Lingshuang Qin, Wei Zhang* and Rui Cao*
Chin. J. Struct. Chem. 2022, 41, 2204022-2204033 DOI: 10.14102/j.cnki.0254-5861.2022-0029
April 8, 2022
water oxidation, Mn-based electrocatalysts, oxygen evolution reaction, structure, mechanism
ABSTRACT
Searching for a renewable energy system is always the goal to fulfill sustainable development for the future. Water oxidation is considered as a crucial reaction to attain sustainable energy systems. Inspired by the biological Mn4CaO5 cluster, considerable effort has been devoted to developing highly efficient Mn-based heterogeneous catalysts and exploring intrinsic mechanism for water oxidation. This review begins with describing the structural characteristics of the Mn4CaO5 cluster and the proposed catalytic cycle. Then, the structural characteristics of synthetic Mn-based heterogeneous catalyst are summarized, with emphasis on the understanding of reaction mechanisms and the rate-determining steps. Finally, the strategy of understanding the catalytic mechanism of Mn-based water oxidation is prospected. -
Communication
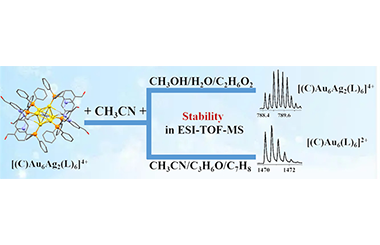
Tuning Solvent Composition to Enhance the Stability of Metal Clusters in Mass Spectrometry
Yingzi Han, Yihuang Jiang, Jing Jeanne Yang*, Shuichao Lin, Zichao Tang* and Lansun Zheng
Chin. J. Struct. Chem. 2022, 41, 2204034-2204039 DOI: 10.14102/j.cnki.0254-5861.2022-0032
April 8, 2022
metal cluster, mass spectrometry, fragmentation, stability, hydrogen bond
ABSTRACT
Electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) has been recognized as a powerful technique for studying metal clusters’ chemical composition and reaction mechanisms. It is a great challenge in mass spectrometry analysis to maintain the metal cluster molecules intact without fragmentation, which is achieved in this work by using mixed solvents to change the interaction between cluster molecules and solvent molecules, further affecting the fragmentation behaviors of the metal cluster in MS. Theoretical analysis reveals that the stability of the [(C)Au6Ag2(C18H14ONP)6]4+ cluster in ESI-TOF-MS is related to the strength of the chemical bonds between its own atoms and the bonding between the solvent and the cluster molecules. -
Communication
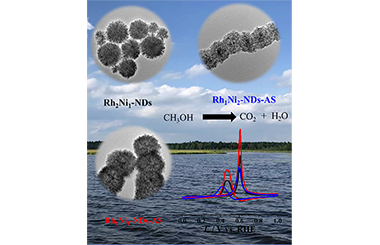
One-Dimensional Rhodium-Nickel Alloy Assemblies with Nano-Dendrite Subunits for Alkaline Methanol Oxidation
Yue Zhao, Yachong Liu, Boqiang Miao, Yu Ding, Pujun Jin* and Yu Chen*
Chin. J. Struct. Chem. 2022, 41, 2204040-2204045 DOI: 10.14102/j.cnki.0254-5861.2022-0019
April 8, 2022
rhodium nanomaterials, fuel cells, methanol oxidation reaction, nanodendrites, alloy
ABSTRACT
The noble metal rhodium (Rh) nanostructures have wide applications in catalysis and electrocatalysis. In this work, a series of RhNi alloy nanostructures with different Rh/Ni atomic ratios can be synthesized by one-step wet chemical method. By adjusting RhIII/NiII feed ratio, high-quality one-dimensional Rh1Ni1 alloy nanodendrite assemblies (Rh1Ni1-NDs-As) can be synthesized selectively. Electrochemical measurements show that the introduction of Ni can enhance the electroactivity of Rh nanomaterials for methanol oxidation reaction (MOR) in alkaline media. For potential practical application, Rh1Ni1-NDs-As reveals improved electroactivity and durability for MOR compared to commercial Rh nanoparticle due to the particular morphology, Ni-doping, high electrochemical active area, and excellent anti-poison ability. Considering the facial synthesis and high electroactivity/durability, Rh1Ni1-NDs-As may be promising anode catalysts in direct methanol fuel cells. -
Article
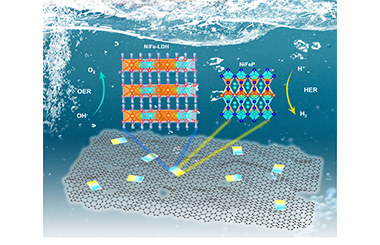
Interfacial Engineering of NiFeP/NiFe-LDH Heterojunction for Efficient Overall Water Splitting
Xuanyu Long, Jiazhi Meng, Jiabao Gu, Lanqing Ling, Qianwen Li, Nan Liu, Kaiwen Wang* and Zequan Li*
Chin. J. Struct. Chem. 2022, 41, 2204046-2204053 DOI: 10.14102/j.cnki.0254-5861.2022-0048
April 8, 2022
overall water splitting, electrocatalysis, heterojunction, phosphide, layered double hydroxide
ABSTRACT
In consideration of application prospect of non-noble metallic materials catalysts, the study of exploring more highly effective electrocatalysts has been focused on by researchers. Herein, a novel strategy is employed to construct a heterojunction consisting of metal phosphide NixFeyP and layered double hydroxide (LDH) with graphene oxide (GO) as conductive support. By adjusting the molar ratio of Ni to Fe, a series of heterojunctions with mixed valence state Feδ+/Fe3+ and Niδ+/Ni2+ (δ is likely close to 0) redox couples are achieved and strong synergistic effects towards overall water splitting performance are found. The optimized catalyst with a Ni/Fe molar ratio of 0.72:0.33, namely Ni0.7Fe0.3P/LDH/GO, delivers ultra-low overpotentials for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) of 79 and 198 mV at the current density of 10 mA·cm-2, respectively. Furthermore, for overall water-splitting practical application, it only requires 1.526 V at 10 mA·cm-2 with robust stability, which is superior to most reported electrocatalysts. Experimental results demonstrate the improved electronic conductivity, enlarged electrochemically active area and accelerated kinetics together account for the enhanced performance. This work supplies new prospects for the promotion and application of such heterojunction electrocatalysts in overall water splitting. -
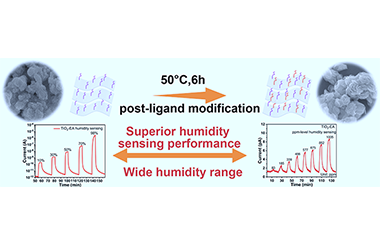
ArticleAtomically Thin 2D TiO2 Nanosheets with Ligand Modified Surface for Ultra-Sensitive Humidity Sensor
Jianze Xiao, Zhihua Fu, Guane Wang, Xiaoliang Ye* and Gang Xu*
Chin. J. Struct. Chem. 2022, 41, 2204054-2204060 DOI: 10.14102/j.cnki.0254-5861.2022-0046
April 8, 2022
TiO2 nanosheet, ligand modification, ultra-sensitive, chemiresistive, humidity sensing
ABSTRACT
As a kind of two-dimensional (2D) nanostructured materials, metal oxide nanosheets (MONS) are attractive and promising humidity sensing materials due to their considerable surface area, good charge carrier transportation, and designable surface functional groups properties. Nevertheless, the ultra-thin MONS modified with active functional groups for humidity sensing are still rare. As a proof of concept, the atomically thin TiO2 nanosheets with high surface area and electron-donating amino groups are prepared by a structure-maintained post-ligand modification strategy. The fabricated TiO2-based sensors demonstrate superior humidity sensing performance with high response, short response time, narrow hysteresis, and ultra-low theoretical limit of detection of about 15 ppm. Additionally, the possible mechanism is proposed from the AC complex impedance measurements and DC instantaneous reverse polarity experiments. This work provides a possible path for developing the high-performance 2D nanostructured metal oxides-based humidity materials through the surface chemical method. -
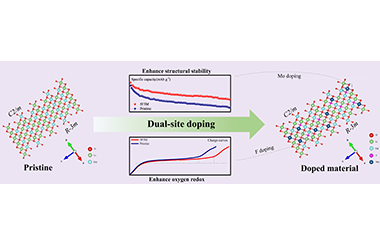
ArticleDual-Site Doping to Enhance Oxygen Redox and Structural Stability of Li-Rich Layered Oxides
Zuhao Zhang, Xiaoyan Xie, Huixian Xie, Xiaokai Ding, Jiaxiang Cui, Chenyu Liu*, Dong Luo* and Zhan Lin
Chin. J. Struct. Chem. 2022, 41, 2204061-2204067 DOI: 10.14102/j.cnki.0254-5861.2022-0066
April 8, 2022
lithium-ion battery, Li-rich layered oxides, doping, Li2MnO3
ABSTRACT
Cobalt-free Li-rich layered oxides (LLOs) such as Li1.2Mn0.6Ni0.2O2 have attracted extensive attention owing to their high specific capacity and low cost. Nonetheless, numerous problems such as continuous voltage fading and capacity decay have become stumbling blocks in its commercial application. In this study, we propose an effective dual-site doping strategy by choosing Mo as the cation and F as the anion to enhance the capacity and cycling performance. The research demonstrates that the cycling stability of LLOs enhances with the doping ratio of Mo, and their capacity increases with the doping ratio of F. It is because Mo as a pillar enhances the structural stability and F doping is conducive to the activation of Li2MnO3. What’s more, dual-site doping also promotes the diffusion of Li+ and reduces the internal resistance of the electrode. Due to these improvements, the 5F3M sample still maintains a discharge capacity of 190.98 mAh g-1 after 100 cycles at 200 mA g-1, which is much higher than 165.29 mAh g-1 of the Pristine sample. This discovery provides a new way to develop advanced layered oxide cathodes for both Na- and Li-ion batteries. -
Article
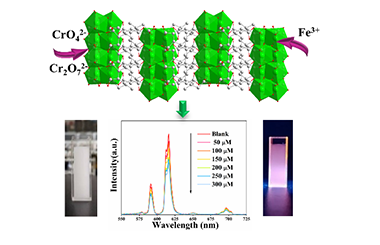
An Ultra-Stable Eu3+ Doped Yttrium Coordination Polymer with Dual-Function Sensing for Cr(VI) and Fe(III) Ions in Aqueous Solution
Fazheng Huang, Xinhao Li, Zhen Zhang, Ziqi Jiang, Guoqiang Wang, Lingyun Li* and Yan Yu*
Chin. J. Struct. Chem. 2022,41, 2204068-2204074 DOI: 10.14102/j.cnki.0254-5861.2021-0071
April 8, 2022
Cr(VI), Fe(III), luminescence sensing, dual-function detection, MIL-92
ABSTRACT
The exploration of a dual-functional sensor is the key to effectively detect the Cr(VI) and Fe(III) cations in water, which is important to human health and environmental sustainability. Because of the phase stability and excellent luminescence, the rare-earth coordination polymers have great potential as dual-functional sensors. Here, we develop a Y0.91Eu0.09(H2O)2{C6H3(CO2)3}(MIL-92(Y):9%Eu3+)-based dual-function luminescent sensor on Cr(VI) and Fe(III), which exhibits excellent phase stability and dispersibility in water. The luminescence of MIL-92(Y):9%Eu3+ aqueous suspension quenches on Fe3+ with Stern-Volmer constant Ksv of 1.79 × 103 M-1 and limit of detection of 17 μM. The MIL-92(Y):9%Eu3+ aqueous suspension also has turn-off sensing ability towards Cr2O72- and CrO42- with Ksv values of 3.5 × 103 and 6.14 × 103 M-1, respectively. It has detection limitations of 10 and 5 μM on Cr2O72- and Cr2O72- ions, respectively.
-
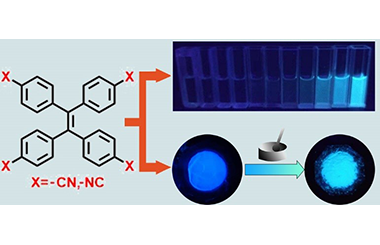
LetterCyano and Isocyano-substituted Tetraphenylethylene with AIE Behavior and Mechanoresponsive Behavior
Qing Liu, Shusheng Yue, Zhangqiang Yan, Yongfa Xie* and Hu Cai*
Chin. J. Struct. Chem. 2022, 41, 2204075-2204082 DOI: 10.14102/j.cnki.0254-5861.2021-0049
April 8, 2022
cyano-group, isocyano-group, tetraphenylethlene (TPE), aggregation induced emission (AIE), mechanochromic
ABSTRACT
Dual-functional materials with AIE behavior and mechanoresponsive behavior have attracted considerable attention due to their promising applications in mechano-sensors, optical storage, solid-state optoelectric devices and bioimage systems. AIEgens bearing tetraphenylethylene (TPE) core become elementary building blocks in many fluorescent functional materials. In this article, cyano and isocyano-electronic withdrawing groups are incorporated with TPE skeleton to form tetracyanophenylethylene (TPE-CN) and tetraisocyanophenylethylene (TPE-NC). Their structures are confirmed by NMR, Mass Spectra and single crystal X-ray measurement. These two isomers reflect aggregation-induced emission (AIE) property in solution state and mechanochromic behavior in solid state. Interestingly, their luminescent intensities, quantum yields and fluorescent lifetime in solid state have an obvious increase upon grinding. The theoretical calculation of these two compounds clarify their difference in optical properties. The mechanochromic mechanism is also intensively explained by powder X-ray measurements.