Yang Yang, Jing-Li Luo, Xian-Zhu Fu*
Chin. J. Struct. Chem., 2024, 43: 100269. DOI: 10.1016/j.cjsc.2024.100269
May 15, 2024
ABSTRACT
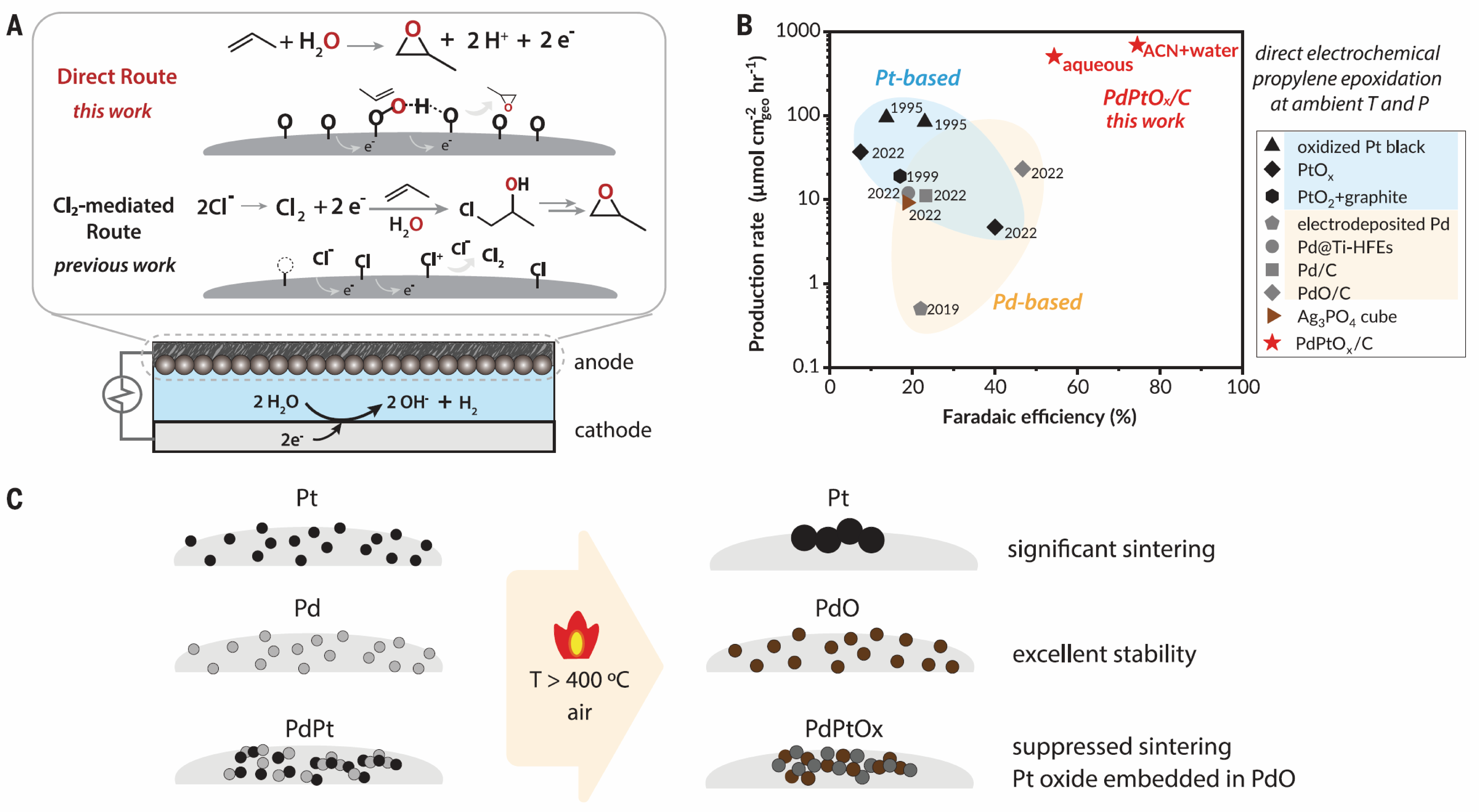
The successful implementation of direct anodic epoxidation relies on the compatibility of electrolyte system with specific catalysts and alkene substrates. In the oxidation of various alkene substrates using a water-acetonitrile mixed electrolyte, acetonitrile enhances the solubility of hydrophobic substrates, but organic solvents may increase the overall resistance of the electrolyte. When water is used as the sole solvent, gas-diffusion electrodes can address the low solubility issue of propylene. Therefore, water electrolyte is sufficient for epoxide production. PdPtOx/C catalyst on a gas-diffusion electrode exhibited an average Faradaic efficiency of approximately 60% for propylene epoxidation at a potential of 1.65 V (vs. SHE).