Cover Picture
Luminescent coordination polymers with mixed carboxylate and triazole ligands for rapid detection of chloroprene metabolite
Yue Mao, Zhonghang Chen, Tiankai Sun, Wenyue Cui, Peng Cheng, Wei Shi* Submit a Manuscript
Luminescent coordination polymers with mixed carboxylate and triazole ligands for rapid detection of chloroprene metabolite
Yue Mao, Zhonghang Chen, Tiankai Sun, Wenyue Cui, Peng Cheng, Wei Shi* Submit a Manuscript
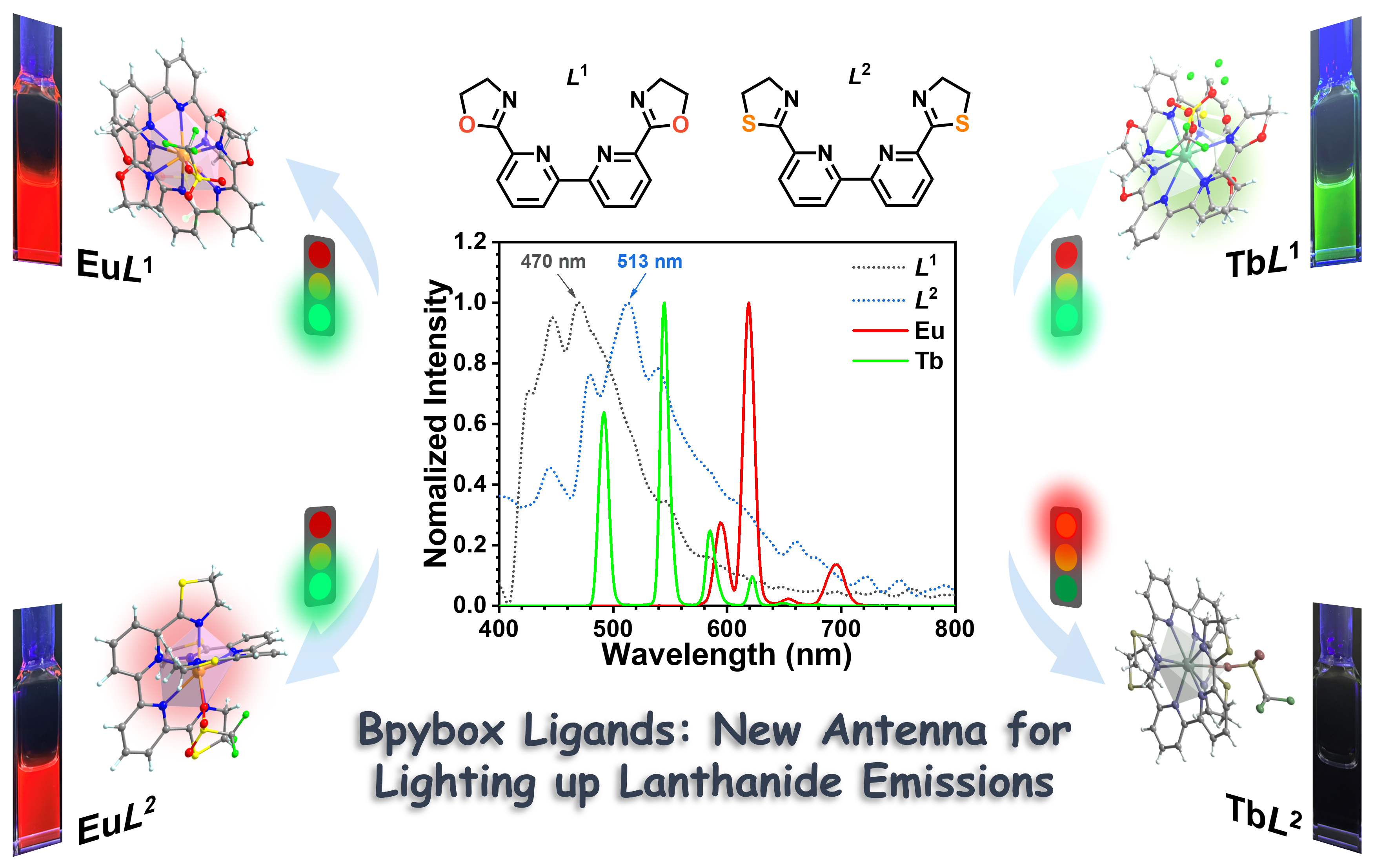
Tuning lanthanide luminescence from bipyridine-bis(oxazoline/thiazoline) tetradentate ligands
Hao Jiang, Yuan-Yuan He, Hai-Chao Liang , Meng-Jia Shang , Han-Han Lu, Chun-Hua Liu, Yin-Shan Meng*, Tao Liu*, Yuan-Yuan Zhu*
Chin. J. Struct. Chem., 2024, 43: 100354. DOI: 10.1016/j.cjsc.2024.100354
September 15, 2024
Lanthanide; Luminescence; Antenna effect; Europium; Terbium
ABSTRACT
Organic ligands play a pivotal role in lanthanide luminescence by acting as sensitizers for energy absorption and transfer, a phenomenon known as the fluorescence antenna effect. Herein, we introduce two tetradentate nitrogen ligands renowned for their efficient sensitization of lanthanide luminescence, with their luminous efficacy finely adjustable through subtle modifications to their structure. Through the synthesis of four Eu(III)/Tb(III) mononuclear complexes via the complexation of ligands 6,6′-bis(4,5-dihydrooxazol-2-yl)-2,2′-bipyridine (bpybox, L1) and 6,6′-bis(4,5-dihydrothiazol-2-yl)-2,2′-bipyridine (thio-bpybox, L2) with europium/terbium trifluorosulfonate, structural analysis reveals that the lanthanide ion coordinates with eight nitrogen atoms from two ligands and one oxygen atom from trifluorosulfonate. Among them, two Eu(III) complexes ([Eu(L1)2(SO3CF3)](SO3CF3)2 EuL1 and [Eu(L2)2(SO3CF3)](SO3CF3)2 EuL2) and one Tb(III) complex ([Tb(L1)2(SO3CF3)](SO3CF3)2 TbL1) exhibit luminosity, displaying characteristic lanthanide metal ion luminescence characterized by high fluorescence quantum yields and prolonged excited state lifetimes. In contrast, TbL2 ([Tb(L2)2(SO3CF3)](SO3CF3)2) is non-luminous, with the sole structural distinction being the substitution of oxygen atoms with sulfur atoms within the ligand. This minor alteration significantly impacts the triplet (3T) state energy level of the ligands, thereby influencing their sensitizing effect on Tb(III) luminescence. These findings underscore the remarkable efficiency of bpybox-type ligands as sensitizers for Ln(III) luminescence, with their structural versatility enabling effective modulation of luminous capacities and efficiency.