Insight into stable, concentrated radicals from sulfur-functionalized alkyne-rich crystalline frameworks and application in solar-to-vapor conversion
Jian-Rong Li, Jieying Hu , Lai-Hon Chung, Jilong Zhou, Parijat Borah, Zhiqing Lin, Yuan-Hui Zhong, Hua-Qun Zhou, Xianghua Yang, Zhengtao Xu*, Jun He*
Submit a Manuscript
Jian-Rong Li, Jieying Hu , Lai-Hon Chung, Jilong Zhou, Parijat Borah, Zhiqing Lin, Yuan-Hui Zhong, Hua-Qun Zhou, Xianghua Yang, Zhengtao Xu*, Jun He*
Chin. J. Struct. Chem., 2024, 43: 100380. DOI: 10.1016/j.cjsc.2024.100380
August 15, 2024
Porous coordination polymers; Persistent radicals; Nanographene; Photothermal conversion; Solar-to-vapor conversion
ABSTRACT
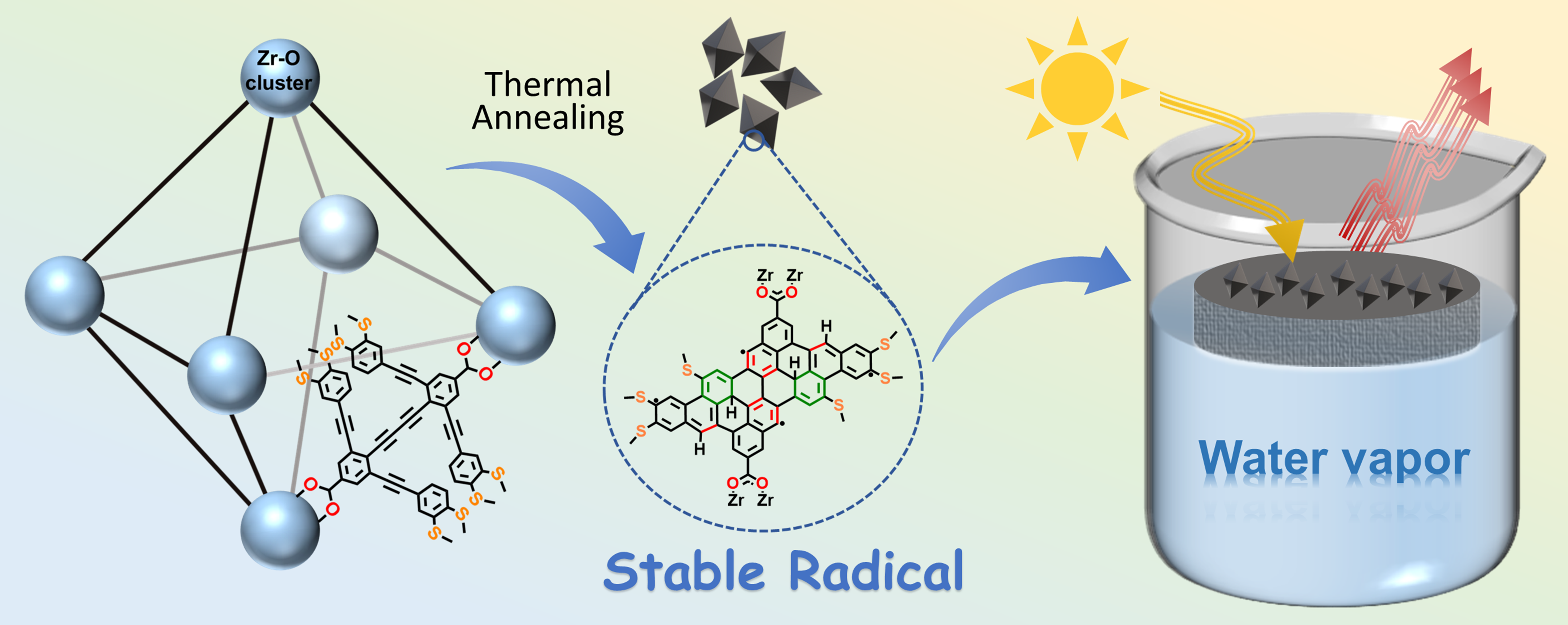
Organic radicals feature versatile unpaired electrons key for photoelectronic and biomedical applications but remain difficult to access in stable concentrated forms. We disclose easy generation of stable, concentrated radicals from various alkynyl phenyl motifs, including 1) sulfur-functionalized alkyne-rich organic linkers in crystalline frameworks; 2) the powders of these molecules alone; 3) simple diethynylbenzenes. For Zr-based framework, the generation of radical-rich crystalline framework was achieved by thermal annealing in the range of 300–450 °C. For terminal alkynes, electron paramagnetic resonance signals (EPR, indicative of free radicals) arise after air exposure or mild heating (e.g., 70 °C). Further heating (e.g., 150 °C for 3 h) raises the radical concentrations up to 3.30 mol kg−1. For more stable internal alkynes, transformations into porous radical solids can also be triggered, albeit at higher temperatures (e.g., 250–500 °C). The resulted radical-containing solids are porous, stable to air as well as heat (up to 300–450 °C) and exhibit photothermal conversion and solar-driven water evaporation capacity. The formation of radicals can be ascribed to extensive alkyne cyclizations, forming defects, dangling bonds and the associated radicals stabilized by polycyclic π-systems.